Abstract
A community of trillions of commensal bacteria inhabit the gastrointestinal tract, collectively known as the intestinal microbiota. The gut microbes are essential for the development and functioning of the enteric nervous system. Approximately two-thirds of the cell bodies of all enteric neurons are gathered in the myenteric plexus, an intricate network of neurons and glial cells that primarily regulate gut neuromuscular activity. Studies in laboratory mice have observed that antibiotic treatment leads to a reduction in microbial abundance and diversity within the intestine, and these findings are correlated with enteric nervous system structural abnormalities. Specifically, antibiotic-treated mice have an abnormal myenteric plexus, which is characterized by a reduction in myenteric neuron numbers and ganglia area. However, it is unknown whether these effects occur in wild Peromyscus mice that are exposed to a natural bacterial flora. The goal of this study was to evaluate the effects of antibiotic exposure on the colonic neurons of the myenteric plexus in wild Peromyscus mice. Thirty-two wild-caught adult male Peromyscus mice were divided into control and antibiotic-treated groups. Whole mount preparations of longitudinal muscle with adherent myenteric plexus were prepared and alterations in colonic neuron and ganglia numbers were assessed by immunohistochemistry analysis. Antibiotic-treatment reduced the total number of colonic enteric neurons/mm2 and the total number of ganglia per myenteric plexus. Our results suggest that antibiotic-induced microbial dysbiosis affects the colonic neurons and ganglia of wild Peromyscus mice similarly to laboratory mice. We showed that the antibiotic-driven changes in neuronal density and ganglia arrangement are inducible in wild Peromyscus species that are exposed to real world bacteria.
Introduction
The gastrointestinal (GI) tract is vital for the digestion of food, the absorption of nutrients and water, and pathogen protection (Heijtz et al., 2011, Obata & Pachnis, 2016, Karl et al., 2018). A community of trillions of commensal bacteria inhabit the GI tract, collectively known as the intestinal microbiota. The gut microbiota is an essential component of the GI tract, co-developing a mutualistic relationship with the host. While the host provides both nutrients and a hospitable environment, the microbiota confers many benefits within the gastrointestinal environment, such as metabolic homeostasis. In addition, the intestinal microbiota influences many aspects of host physiology beyond the GI environment itself, including the development and regulation of nervous system structure and activity (Heijtz et al., 2011, Obata & Pachnis, 2016, Karl et al., 2018).
Intestinal neuromuscular activity, secretory and vasomotor control, and gastric peristalsis are primarily controlled by the enteric nervous system (ENS); a major branch of the autonomic nervous system located throughout the entirety of the GI tract (Hyland & Cryan, 2016). This intricate neuronal system consists of millions of enteric nerve cells embedded within the GI tract. The cells bodies of the enteric neurons are gathered into 2 distinct plexi: the myenteric and submucosal plexus. The submucosal plexus is located underneath the submucosa and is fundamental to the regulation of mucosal functions. The myenteric plexus (MP) is located between the circular and longitudinal muscle layers of the gut and contains up to two-thirds of all enteric neurons. The neurons’ primary function is to regulate GI neuromotor control (Hyland & Cryan, 2016).
The intestinal microbiota is essential to the proper functioning and integrity of the ENS. [2] Multiple studies have observed structural and functional abnormalities of the ENS in response to microbial depletion (Kabouridis & Pachnis, 2015, Neufeld et al., 2015). The ENS can respond to the microbiota and its components via pattern recognition receptors, particularly toll-like receptors (TLRs). Specifically, TLRs recognize microbial derived components which may stimulate various downstream processes that could influence the structural and functional integrity of the ENS via TLR stimulation (Hyland & Cryan, 2016).
Environmental stressors can cause microbial dysbiosis, a shift in the composition of the microbial communities, which consequently alter the internal GI environment (Karl et al., 2018, Galley & Bailey, 2014). Using antibiotics is a common method to induce artificial microbial dysbiosis in laboratory settings as it produces a germ-free (GF) like phenotype, which allows for the exploration of the effects of the microbiota on ENS structure and function (Yoon & Yoon, 2018). Antibiotic administration leads to a reduction in gut flora diversity and abundance. These alterations in the microbial community may impact the interactions of the microbes with the intestinal environment, possibly altering GI function (Membrez et al., 2008, Vijay-Kumar et al., 2010). In particular, microbial dysbiosis from antibiotic treatment is correlated with macroscopic changes in the intestine and functional gut consequences, primarily GI dysmotility (Caputi et al., 2017, Cao et al., 2017). The functional deficiencies associated with microbial dysbiosis may be due to ENS structural alterations as observed by various studies in GF and antibiotic-treated laboratory mice (Caputi et al., 2017, Hyland & Cryan, 2016). Microbial depletion and dysbiosis are associated with alterations in the MP (Caputi et al., 2017, Collins et al., 2014). These studies highlight the importance of the intestinal microbiota in ENS structural and functional integrity.
In the present study, we sought to evaluate if these structural alterations in the ENS observed in antibiotic-treated laboratory mice translate to wild Peromyscus species. Wild mice are exposed to natural bacteria and thus have greater gut flora diversity in comparison with multi-generational laboratory mice that are kept in specific pathogen-free facilities for most of their lives (Wang et al., 2014). Conventional laboratory mice have limited translational research value because they lack real world gut microbe diversity, and so they may not faithfully mirror the physiology of wild species (Rosshart et al., 2019). However, wild mice populations contain natural microbiota and thus may better recapitulate free-living species, including humans (Rosshart et al., 2019).
The present study was designed to evaluate the effects of antibiotics on the colonic neurons of the MP in wild Peromyscus mice. It was hypothesized that antibiotic-induced dysbiosis of the gut microbiota would alter the number of colonic neurons within the MP. Specifically, it was predicted that microbial dysbiosis will reduce the number of colonic neurons, which is consistent with findings in laboratory mice.
Methods
Trapping
Wild Peromyscus species were trapped in deciduous forests and abandoned barns around Trent University. The mice were sexed. Adult males were individually housed in a thermoneutral and humidity-controlled room.
Experimental Design
The mice were housed for a minimum of 7 days and weighed approximately 18g before being separated into treatment groups. They were separated into two primary treatment groups: Antibiotics (n=20) and Control (n=20). Antibiotic treated mice were given broad spectrum antibiotics (0.5g/L Neomycin Sigma N1876, Lot: WXBB7516V and 1.0g/L Ampicillin Sodium salt Sigma A9518, Lot: 085M4953V) administered through their drinking water and replenished weekly. The experiment duration ran from weeks 0 to 6 monitoring body weight, food intake, and water intake were measured weekly as previously described (Garnett, 2016). Fecal samples were collected from the cages at weeks 1 and 3. At week 6, the mice were euthanized.
Intestinal Dissection and Preparation for Immunohistochemistry
The intestine was removed starting at the lower esophageal sphincter to the rectum. The large intestine segment was separated by cutting the tissue 1-inch below the cecum. The large intestine segments were cut longitudinally at the mesentery, stretched, and pinned out on sylgard resin plates and fixed with 4% paraformaldehyde (replaced with PBS). Using a dissection microscope (LEICA ES2 Stereo Microscope), whole mount preparations consisting of the longitudinal muscle with the myenteric plexus attached (LMMPs) were prepared by carefully removing the mucosal, submucosal, and circular muscle layers. Whole mount preparations were all prepared by the same person to reduce variability.
Cecum Content Culture
Alterations in microbiota were determined through culturing cecum content as previously described (Garnett, 2016). The contents of the cecum were collected during dissection; 0.04g were placed into 1mL nutrient buffer (1g/50mL), mixed, and placed in a 4°C fridge overnight. Cecal contents were placed at room temperature 2 hours prior to culturing. Culturing was completed on both nutrient agar (Thermo Fisher Scientific, lot: 1800844, 0.028g/mL) and blood agar (0.028g/mL nutrient agar with 0.05mL of sheep’s blood per milliliter agar lot: 332503) (Chevelier et al. 2015). 20μL of the nutrient buffer with cecum content solution was placed on each plate. Nutrient buffer with no cecum content added was used as a control sample. The nutrient agar plates were kept in an aerobic chamber at 37°C and the blood agar plates were kept in an anerobic chamber at 37°C. All plates were left in the chamber for 48 hours before recording the approximate abundance of all colonies (Garnett, 2016).
Immunohistochemistry on Colonic Whole-Mount Preparations
The whole mount preparations of LMMP were blocked with PBS-Triton with 4% 100 level blocking serum (Stanbio, Lot: 14860) for 2 hours with gentle shaking every 15 – 20 minutes to reduce non-specific binding of the primary antibody. Next, the LMMP was incubated overnight (21 hours) at 4°C in a 1:1000 concentration of primary antibody HuR (6A97) monoclonal Mouse IgG (Santa Cruz Biotechnology, 71290, lot: C0713) diluted in PBS-Triton with 4% 100 level blocking serum. The primary antibody HuR is a pan-neuronal marker that is specific for HuR, an RNA-binding protein that is found within the nucleus and cytoplasm of all neurons (Phillips, Hargrave, Rhodes, Zopt & Powley, 2003). Following primary antibody incubation, the tissue samples were rinsed sequentially in PBS 3 times for 5 minutes each to remove unbound primary antibody. The tissue samples were then incubated for 2 hours at room temperature in a 1:500 dilution of goat anti-mouse IgG-FITC secondary antibody (Santa Cruz Biotechnology, sc-2010, lot: G2314) in PBS-Triton with 4% 100 level blocking serum. After secondary incubation, the tissue samples were rinsed sequentially in PBS 3 times for 5 minutes each. LMMP samples were then mounted on glass slides using mounting medium (UltraCruz, Lot: HH0813) for fluorescence microscopy (LEICA DFC350 FX, Leica application suite 2.4.1 build 6384).
Acquisition of Images
Images were acquired with a LEICA DFC3650 FX CCD microscope camera using a LEICA DFC3550 FX epifluorescence microscope equipped with a high magnification 40x/1.4 objective and a low magnification 10x/0.22 objective. The IgG-FITC secondary antibody has an emission peak of 525mm (Santa Cruz Biotechnology). Before image acquisition and analysis, all tissue preparations were coded, and the investigator was blinded to the experimental conditions.
Images of each LMMP sample were acquired at both 10X magnification and 40X magnification. Regions of the LMMP with large tears or sections of MP that had been removed during dissection were omitted because they were deemed to be non-representative of the overall tissue. At 10X magnification, images were taken randomly throughout the tissue. These images would be used to determine neuron and ganglion numbers. At 40X magnification, the microscope camera was used to randomly focus on and take pictures of individual ganglions. These images would be used to determine the number of neurons within a single ganglion.
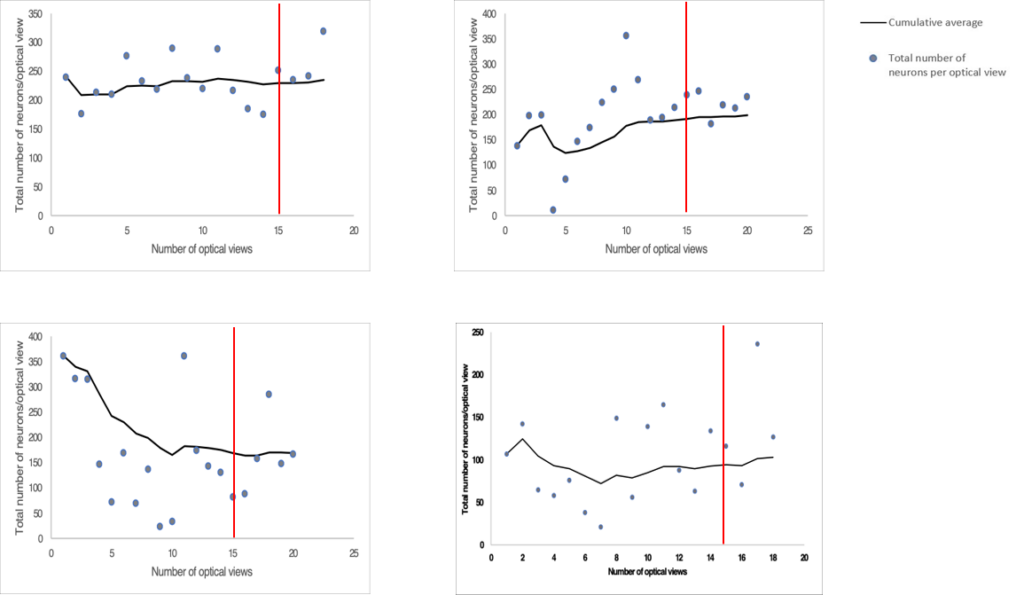
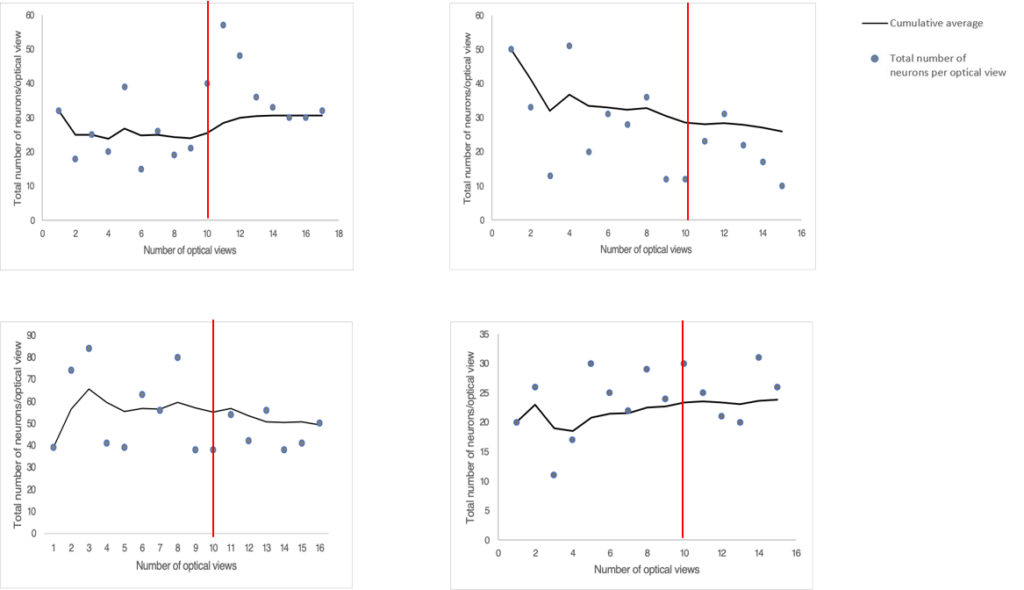
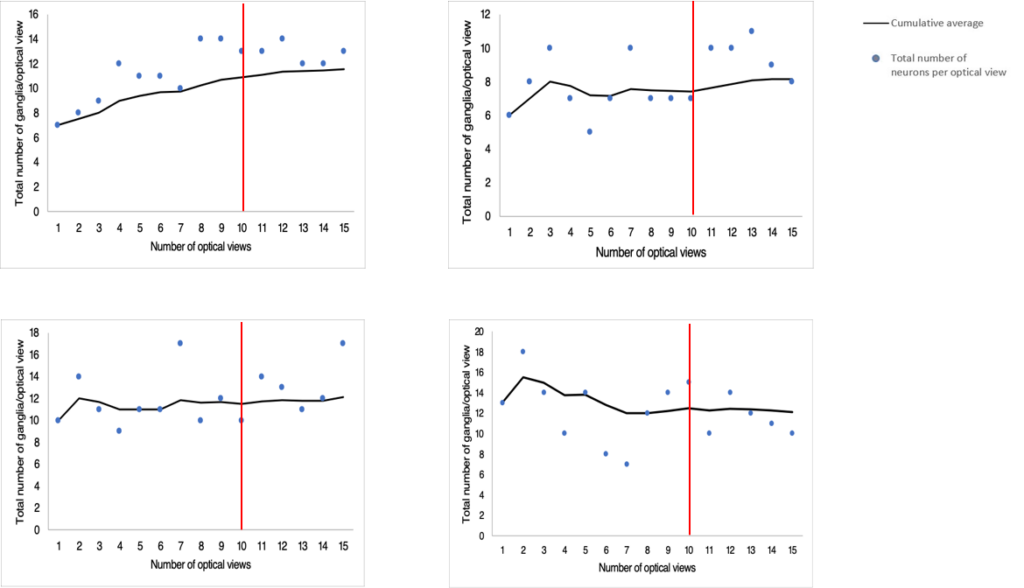
A pilot study was conducted to determine the minimum number of images that would need to be captured per tissue at each magnification to get a representative estimate of the total neuron and ganglia populations in each LMMP tissue. Four randomly selected tissues were chosen for the pilot study. We captured as many microscope fields as possible at both 10X and 40X magnification. Approximately 20 images were acquired per tissue at each magnification. The total number of myenteric neurons and ganglia were counted at both 10X and 40X magnification and expressed as a cumulative average. We plotted the cumulative averages from each tissue count and observed the trends until they reached a plateau. We estimated when we had imaged enough microscope fields when the cumulative average remained relatively flat without any upward or downwards trends.
We determined that we would need to capture 15 images per tissue at 10X magnification for total neuron counts (Figure S2). From these 15 images, we would need to use 10 images for total ganglia counts (Figure S3). We would need to capture 10 images of randomly selected individual ganglions at 40X magnification for total neuron counts per ganglion (Figure S1).
Data Analysis
All images were imported into ImageJ to be counted using the “Cell Counting and Marking” plugin. This plugin automatically counts all manually labelled neurons. By labelling a neuron we ensured that each cell was only counted once. Neurons were defined as green fluorescent immunolabeled HuR-positive cells. Neurons bodies that were not completely within the microscope’s field of view were only counted if more than 50% of their body was visible. In addition, ganglia were defined as clearly delineated groups of neurons separated by noticeable nerve fibre tracts. If the ganglia were not gathered into noticeable clusters, we defined their boundaries as an area where connecting strands were smaller than the width of 2 neurons. This ganglion definition has previously been described in a similar study (Stenkamp-Strahm, Kappmeyer, Schmalz. Gericke & Balemba, 2013). When counting total neurons within an individual ganglion, we did not include neurons located outside the delineated ganglion structure.
Total neuron and ganglion numbers were quantified and expressed in a variety of ways:
- Total neuron number per MP was quantified by counting the total amount of neurons in each of the 15 images acquired at 10X magnification per tissue.
- Total neuron number was expressed in two ways:The total neuron number was expressed as a cumulative mean count per MP.
- The data was normalized to a total area of 16.8mm2 (total field of view area at 10X magnification) and expressed as a cumulative mean neuron count per total field of view area (mm2).
- Total ganglia number per MP was quantified by counting the total amount of ganglia in each of the 10 images acquired at 10X magnification per tissue. The total ganglia number was expressed as a cumulative mean count per MP.
- Total neurons per ganglia was quantified in two ways:
- Direct quantification: Total neuron number per ganglia was quantified by counting the total amount of neurons in a single ganglion in each of the 10 images acquired of individual ganglions at 40X magnification per tissue.
- Indirect quantification: Total neuron number per ganglia was calculated by dividing the total number of neurons by the total number of ganglia counted per tissue using 10X magnification counts above.
Both quantification methods were expressed as a cumulative mean neuron count per myenteric ganglia.
Statistical Analysis
All results are presented as a mean ± standard error of the mean (SEM). Statistical significance was calculated with an unpaired Student’s t-test for two-sample comparisons and univariant general linear model for multiple comparisons. Differences between groups were considered significant when P <0.05. N values indicate the number of tissues used per group. One tissue was used per mouse. Discarded tissues were not included.
Results
Consistent body size and weight
There was no difference in mean body weights before the start of the experiment between treatment and control groups. There was no change in body weight from week 0 to week 6 between antibiotic-treated and control mice. Antibiotic administration did not cause a change in food consumption.
Antibiotics quantitatively alter cultured bacterial growth
Cecum bacteria were grown to quantitatively assess colony abundance between antibiotic-exposed and control groups. Antibiotic treatment led to increased bacterial culture growth when compared to control mice (Garnett, 2016).
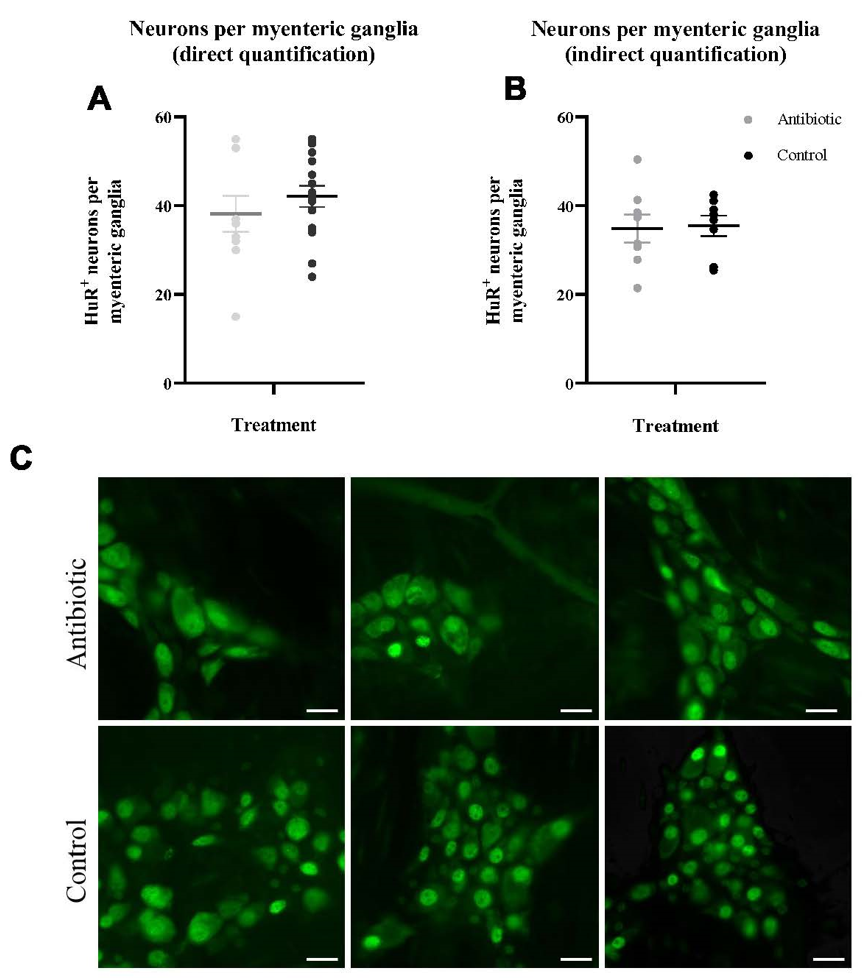
Antibiotics do not alter total neurons per myenteric ganglia
Antibiotic administration in wild Peromyscus mice did not lead to a significant reduction in the average number of total neurons per myenteric ganglia using either quantification method; direct quantification at 40X magnification (p=0.21) or indirect quantification calculated from data at 10X magnification (p=0.44) (Figure 1A-B).
Antibiotics reduce the total number of enteric neurons in the myenteric plexus
We found that antibiotic treatment in wild Peromyscus mice led to a 24.5% reduction in the average number of total neurons per field of view area (mm2) (p=0.04) from a mean of 20.33 total neurons in the control group to a mean of 15.35 total neurons in the antibiotic treated group. In addition, we observed that the average number of total neurons per MP showed a similar trend, though not statistically significant (p=0.09) (Figure 2A-B). P<0.05 is significantly different from control.
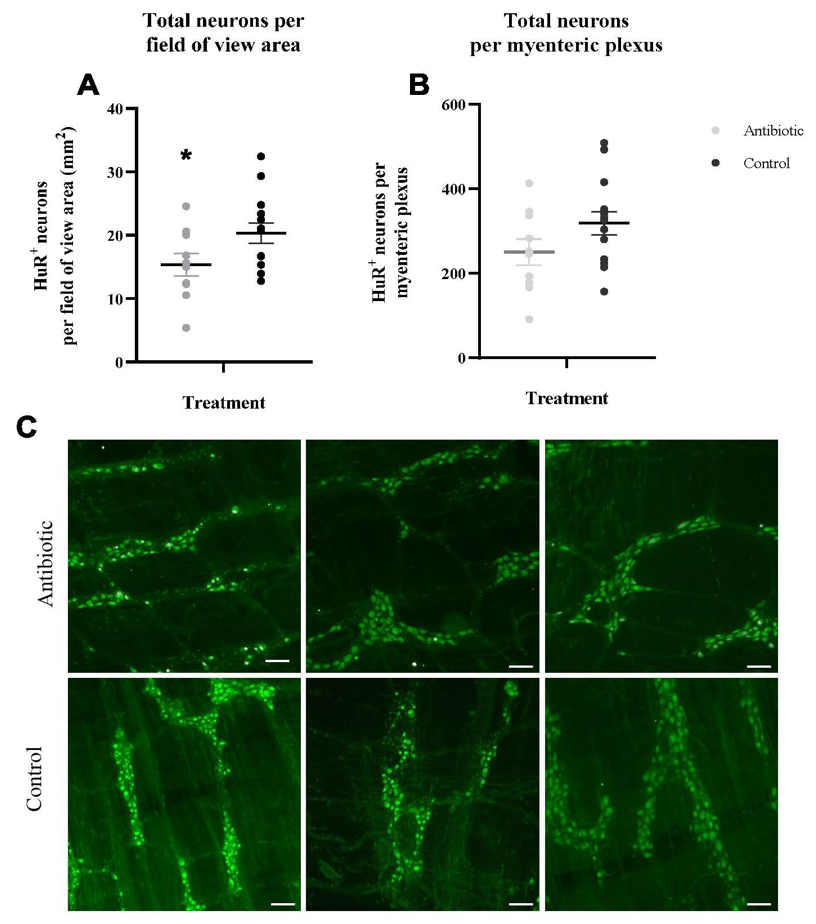
Antibiotics reduce the total number of ganglia in the myenteric plexus
We discovered that antibiotic treatment in wild Peromyscus mice led to a 17.7% reduction in the average number of total ganglia per MP (p=0.05) in comparison to the control mice (Figure 3A).
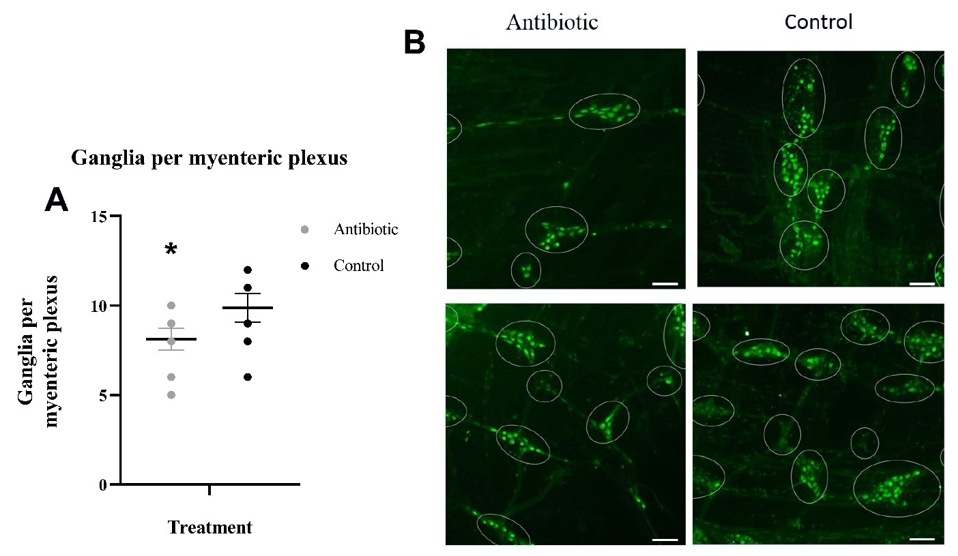
Discussion
In the present study, we demonstrated the effects of antibiotic treatment on the colonic neurons of the MP in wild Peromyscus mice. This study demonstrated that antibiotic-induced dysbiosis has the following effects: 1) non-significant but trending reduction in the total number of neurons per myenteric ganglia; 2) reduction in the total number of neurons per field of view area (mm2); 3) non-significant but trending reduction in total neurons per MP; and 4) a reduction in the total number of ganglia per MP.
It is assumed that the gut microbiota diversity in the Peromyscus mice was altered by the antibiotics, ampicillin and neomycin, similar to lab mice that had received the same doses in previously published studies (Cani et al. 2008). These studies found a 44% similarity in the cecal bacterial communities between antibiotic-treated and control mice. Our cultures of cecal contents from antibiotic treated mice had increased bacterial growth (Garnett, 2016). The broad-spectrum antibiotics may have created opportunities for bacterial growth of remaining bacteria that are normally restricted by microbial competition (Rea et al. 2011).
We observed a significant reduction in the total number of colonic enteric neurons per field of view area in the antibiotic administered mice as suggested by previous studies (Collins et al., 2014, Caputi et al., 2017). We suggest that the observed decreases in total neurons may be due to an antibiotic-induced reduction in microbial abundance and diversity, which may in turn affect colonic neurons. These findings are consistent with data from GF and toll-like receptor (TLRs) knockout mice (Anitha et al., 2012, Brun et al., 2013, Caputi et al., 2017). It is thought that microbial dysbiosis or depletion may be interfering with the cross-talk between microbes and the ENS, and this bi-directional communication may be mediated by pattern recognition receptors, namely TLRs that are capable of directly responding to microbial-derived products. It has been suggested that the maintenance of low doses of lipopolysaccharide, a gram-negative bacterial derived product, may be important in maintaining neuronal survival in adult enteric neurons via TLR stimulation (Anitha et al., 2012). Thus, the structural abnormalities in TLR knockout mice may be associated with a loss in TLR signalling (Anitha et al., 2012). Similarly, microbial depletion from antibiotic treatment may also be interfering with TLR signalling. Our results suggest that these disruptions in TLR signalling could explain the neuronal loss observed in antibiotic-treated Peromyscus mice. Other studies have also observed a decrease in the number of neurons per myenteric ganglia in mice treated with antibiotics (Collins et al., 2014, Caputi et al., 2017). Although we did not see a significant reduction in the total number of enteric neurons per myenteric ganglia, the present study shows that the decline in total neuron numbers per ganglia is trending in this expected direction.
We observed a marked decrease in the total number of ganglia per MP in the antibiotic administered mice. We suggest that this may also be due to reduction in microbial diversity and abundance from the antibiotic treatment. In previous studies, microbial-depletion has been associated with an abnormal MP pattern in GF mice (Collins et al., 2014). Normally, the MP is organized in a stereotypical meshwork of evenly spaced ganglia and interconnecting nerve strands of even thickness. However, in GF mice the MP is less organized, has less abundant nerve fibers, and has unevenly spaced and smaller ganglia compared to conventionally colonized mice (Collins et al., 2014). In addition, a specific marker for enteric ganglion cells, peripherin, has been observed to have an altered expression and distribution in the myenteric ganglia of GF mice, in correlation with a reduction in myenteric ganglia area. Peripherin is a neurofilament protein that forms an important part of the cytoskeleton of various enteric ganglion cells and is often associated with smaller ganglia areas (Brun et al., 2013). We suggest that the microbiota may interact with the ENS to influence ganglia growth and survival via similar mechanisms to those mediating neuronal growth as proposed earlier. Particularly, the administration of the neurotrophic factor, glial-cell derived neurotrophic factor (GDNF) seems to restore peripherin expression levels in the MP and this is correlated with a partial restoration in ganglia size (Brun et al., 2013). Additionally, GDNF has been observed to promote axonal outgrowth and enteric neuronal aggregation in myenteric neuron cultures, mimicking the appearance of ganglions and their associated nerve tracts in vitro (Rodrigues, Li, Nair, & Blennerhassett, 2010). Thus, GDNF is thought to be an important factor in the growth and survival of the myenteric ganglia (Rodrigues, Li, Nair, & Blennerhassett, 2010).
This study has limitations that should be considered when interpreting the results. First, although all attempts were made to acquire microphotographs of whole mount preparations at identical time conditions, preferably after completion of immunohistochemistry, it was difficult to capture all images at a constant time interval. Thus, there may be subtle variations in staining intensity between tissues due to differences in fluorescence degradation over time. This may have affected the quality and clarity of the images acquired days after completion of immunohistochemistry, making it harder to subjectively discriminate among neurons when manually counting those images. In addition, it is important to set microscope settings to capture all images at a standardized minimum baseline fluorescence to reduce unwanted background noise and fluorescence bleed-throughs which can make it hard to distinguish one neuron from another (Stenkamp-Strahm, Kappmeyer, Schmalz, Gericke & Balemba, 2013). Although efforts were made to capture all images using minimum baseline fluorescence settings, it was hard to keep these settings constant for all images due to of differences in staining intensity between tissues. This also may have affected both the the clarity of the images and the manual counting of neurons. Secondly, we did not include immunohistochemistry control experiments, which were imperative to ensure that the antibody staining is accurate and to rule out endogenous staining (Kim, Roh, & Park, 2016). We may have unknowingly included false-positive results during our neuron counts which could have resulted in an overestimation of neuron numbers. Thus, future studies should prepare control immunohistochemistry experiments by omitting the primary antibody in order to rule out artificial background staining from unspecific secondary antibody binding as previously described (Caputi et al., 2012). Finally, all our images were quantified by only one investigator. Although, we were able to re-count all images to ensure consistency in the neuron counts, it is difficult to ensure accuracy in our total count estimates. Future studies may benefit from having 2 or 3 investigators independently counting the neurons and averaging the estimates across the investigators to ensure more accuracy in neuronal counts as previously mentioned (Phillips, Hargrave, Rhodes, Zopt & Powley, 2004).
In conclusion, we demonstrated the effects of antibiotic treatment on the colonic neurons of the myenteric plexus in wild Peromyscus mice. Particularly, this study demonstrated that antibiotic-induced dysbiosis is associated with a reduction in the total number of enteric neurons per field of view area, and a similar trend was observed in the total number of neurons per myenteric plexus and per myenteric ganglia. Reductions were also observed in the total number of ganglia in the antibiotic-treated mice. Our results suggest that antibiotic-induced microbial dysbiosis affects the colonic neurons and ganglia of wild Peromyscus mice similar to laboratory rodents.
Acknowledgements
The author would like to thank Dr Kirk Hillsley, Dr Holly Bates, Debbie Lietz, and Smolly Coulson “for their guidance and mentorship throughout the project“.
References
- Anitha, M., Vijay-Kumar, M., Sitaraman, S. V., Gewirtz, A. T., & Srinivasan, S. (2012). Gut microbial products regulate murine gastrointestinal motility via Toll-like Receptor 4 signaling. Gastroenterology, 143(4), 1006-1016.e4. https://doi.org/10.1053/j.gastro.2012.06.034
- Brun, P., Giron, M. C., Qesari, M., Porzionato, A., Caputi, V., Zoppellaro, C., … Castagliuolo, I. (2013). Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology, 145(6), 1323–1333. https://doi.org/10.1053/j.gastro.2013.08.047
- Cani, P.D., R. Bibiloni, C. Knauf, A. Waget, A.M. Neyrinck, N.M. Delzenne et al. (2008). Changes in gut microbiota control metabolic endotoxemia induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57(6): 1470-1481.
- Cao, H., Liu, X., An, Y., Zhou, G., Liu, Y., Xu, M., … Wang, B. (2017). Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Scientific Reports, 7. https://doi.org/10.1038/s41598-017-10835-8
- Caputi, V., Marsilio, I., Cerantola, S., Roozfarakh, M., Lante, I., Galuppini, F., … Giron, M. C. (2017). Toll-Like Receptor 4 Modulates Small Intestine Neuromuscular Function through Nitrergic and Purinergic Pathways. Frontiers in Pharmacology, 8. https://doi.org/10.3389/fphar.2017.00350
- Caputi, V., Marsilio, I., Filpa, V., Cerantola, S., Orso, G., Bistoletti, M., … Giron, M. C. (2017). Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. British Journal of Pharmacology, 174(20), 3623–3639. https://doi.org/10.1111/bph.13965
- Collins, J., Borojevic, R., Verdu, E. F., Huizinga, J. D., & Ratcliffe, E. M. (2014). Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterology & Motility, 26(1), 98–107. https://doi.org/10.1111/nmo.12236
- Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701-.
- Delungahawatta, T., Amin, J. Y., Stanisz, A. M., Bienenstock, J., Forsythe, P., & Kunze, W. A. (2017). Antibiotic Driven Changes in Gut Motility Suggest Direct Modulation of Enteric Nervous System. Frontiers in Neuroscience, 11. https://doi.org/10.3389/fnins.2017.00588
- Furness, J. B. (2006). Structure of the enteric nervous system. In The Enteric Nervous System (pp. 1-28). Malden, MA: Blackwell Publishing.
- Galley, J. D., & Bailey, M. T. (2014). Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes, 5(3), 390–396. https://doi.org/10.4161/gmic.28683
- Garnett, L. (2016). Alterations of the gut microbiome by antibiotics accentuates cold induced gut growth but lowers adiposity independent of temperature in Peromyscus (Unpublished thesis). Trent University.
- Heijtz, R. D., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., … Pettersson, S. (2011). Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences, 108(7), 3047–3052. https://doi.org/10.1073/pnas.1010529108
- Hyland, N. P., & Cryan, J. F. (2016). Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Developmental Biology, 417(2), 182–187. https://doi.org/10.1016/j.ydbio.2016.06.027
- Heuckeroth, R. O., & Schäfer, K.-H. (2016). Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention. Developmental Biology, 417(2), 188–197. https://doi.org/10.1016/j.ydbio.2016.03.017
- Kabouridis, P. S., & Pachnis, V. (2015). Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. The Journal of Clinical Investigation, 125(3), 956–964. https://doi.org/10.1172/JCI76308
- Karl, J. P., Hatch, A. M., Arcidiacono, S. M., Pearce, S. C., Pantoja-Feliciano, I. G., Doherty, L. A., & Soares, J. W. (2018). Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Frontiers in Microbiology, 9. https://doi.org/10.3389/fmicb.2018.02013
- Kashyap, P. C., Marcobal, A., Ursell, L. K., Larauche, M., Duboc, H., Earle, K. A., … Sonnenburg, J. L. (2013). Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology, 144(5), 967–977. https://doi.org/10.1053/j.gastro.2013.01.047
- Kennedy, E. A., King, K. Y., & Baldridge, M. T. (2018). Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Frontiers in Physiology, 9. doi:10.3389/fphys.2018.01534
- Kim, S.-W., Roh, J., & Park, C.-S. (2016). Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. Journal of Pathology and Translational Medicine, 50(6), 411–418. https://doi.org/10.4132/jptm.2016.08.08
- Kulkarni, S., Micci, M.-A., Leser, J., Shin, C., Tang, S.-C., Fu, Y.-Y., … Pasricha, P. J. (2017). Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 114(18), E3709–E3718. https://doi.org/10.1073/pnas.1619406114
- Liang, C., Wang, K., Yu, Z., & Xu, B. (2016). Development of a novel mouse constipation model. World Journal of Gastroenterology, 22(9), 2799. doi:10.3748/wjg.v22.i9.2799
- Liang, C., Wang, K., Xu, B., & Yu, Z. (2016). Electroacupuncture at acupoint ST 37(Shangjuxu) improves function of the enteric nervous system in a novel mouse constipation model. BMC Complementary and Alternative Medicine,16(1). doi:10.1186/s12906-016-1377-5
- Neufeld, K. A. M., Mao, Y. K., Bienenstock, J., Foster, J. A., & Kunze, W. A. (2013). The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterology & Motility, 25(2), 183-e88. https://doi.org/10.1111/nmo.12049
- Obata, Y., & Pachnis, V. (2016). The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology, 151(5), 836–844. https://doi.org/10.1053/j.gastro.2016.07.044
- Phillips, R. J., Hargrave, S. L., Rhodes, B. S., Zopf, D. A., & Powley, T. L. (2004). Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. Journal of Neuroscience Methods, 133(1), 99–107. https://doi.org/10.1016/j.jneumeth.2003.10.004
- Quigley, E. M. M. (2011). The enteric microbiota in the pathogenesis and management of constipation. Best Practice & Research Clinical Gastroenterology, 25(1), 119–126. https://doi.org/10.1016/j.bpg.2011.01.003
- Rea, M. C., Dobson, A., O’Sullivan, O., Crispie, F., Fouhy, F., Cotter, P. D., … Ross, R. P. (2011). Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proceedings of the National Academy of Sciences, 108(Supplement 1), 4639. https://doi.org/10.1073/pnas.1001224107
- Rodrigues, D. M., Li, A. Y., Nair, D. G., & Blennerhassett, M. G. (2010). Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterology & Motility, 23(2). doi:10.1111/j.1365-2982.2010.01626.x
- Rosshart, S. P., Herz, J., Vassallo, B. G., Hunter, A., Wall, M. K., Badger, J. H., … Rehermann, B. (2019). Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science, 365(6452). https://doi.org/10.1126/science.aaw4361
- Stenkamp-Strahm, C. M., Kappmeyer, A. J., Schmalz, J. T., Gericke, M., & Balemba, O. (2013). High-fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell and Tissue Research, 354(2), 381–394. https://doi.org/10.1007/s00441-013-1681-z
- Soret, R., Chevalier, J., De Coppet, P., Poupeau, G., Derkinderen, P., Segain, J. P., & Neunlist, M. (2010). Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology, 138(5), 1772-1782.e4. https://doi.org/10.1053/j.gastro.2010.01.053
- Sun, J., & Dudeja, P. K. (2018). Mechanisms Underlying Host-Microbiome Interactions in Pathophysiology of Human Diseases. Springer.
- Uesaka, T., Young, H. M., Pachnis, V., & Enomoto, H. (2016). Development of the intrinsic and extrinsic innervation of the gut. Developmental Biology, 417(2), 158–167. https://doi.org/10.1016/j.ydbio.2016.04.016
- Vijay-Kumar, M., Aitken, J. D., Carvalho, F. A., Cullender, T. C., Mwangi, S., Srinivasan, S., … Gewirtz, A. T. (2010). Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science (New York, N.Y.), 328(5975), 228–231. https://doi.org/10.1126/science.1179721
- Wang, J., Kalyan, S., Steck, N., Turner, L. M., Harr, B., Künzel, S., … Baines, J. F. (2015). Analysis of intestinal microbiota in hybrid house mice reveals evolutionary divergence in a vertebrate hologenome. Nature Communications, 6, 6440. https://doi.org/10.1038/ncomms7440
- Yoon, M. Y., & Yoon, S. S. (2018). Disruption of the Gut Ecosystem by Antibiotics. Yonsei Medical Journal, 59(1), 4–12. https://doi.org/10.3349/ymj.2018.59.1.4
