Abstract
The scarcity of iron in marine environments, particularly in the Southern Ocean, provides ideal experimental grounds for large-scale iron fertilization. The Iron Hypothesis, proposed in the 1980s, sparked a series of in-situ fertilization experiments in the HNLC waters of the Southern Ocean aiming to prove that the addition of Fe(II) to iron-deficient phytoplankton populations would enhance photosynthetic processes to the point where they could serve as a viable method of mitigating anthropogenic carbon emissions through the acceleration of oceanic carbon sequestration. By examining the mechanisms behind iron fertilization, as well as contributions to the field and the gaps in knowledge pertaining to three major iron fertilization experiments – SOIREE, SOFeX, and LOHAFEX – this review will provide a comprehensive overview of the past, present, and future of iron fertilization.
Introduction
Iron is an essential nutrient in key biological processes such as amino acid synthesis, oxygen transport, respiration, nitrogen fixation, the citric acid cycle, and photosynthesis. Obtaining iron, however, presents a challenge particularly for microorganisms living in iron-limited environments (Butler & Sandy, 2009). While iron is the 4th most abundant transition metal on the planet, the insolubility of Fe(III) (ferric iron – Ksp of Fe(OH)3 = 6.0 x 10-38) and Fe(II) (ferrous iron – Ksp of Fe(OH)2 = 2.0 x 10−15) at physiological pH in aerobic environments severely limits the biological availability of this essential nutrient (Sillén et al., 1964). Marine organisms face a greater challenge than terrestrial organisms, as seawater contains 0.001-0.003 ppm of available iron in comparison to 0.5-1 ppm in river water and 100 ppm in groundwater (Lenntech, n.d.). Despite low oceanic iron concentrations, phytoplankton are the world’s most influential primary photosynthetic producers, producing an estimated 50-85% of the planet’s oxygen (Lin et al., 2003). It follows that phytoplankton are capable of intaking large volumes (30-50%) of the world’s carbon dioxide (CO2) to carry out photosynthetic processes, thereby transforming the ocean into the largest carbon sink on the planet (Block, 2018; The Earth Institute, 2009).
It had been determined by 1980 that phytoplankton photosynthesis is limited by iron bio-availability, as opposed to other essential nutrients. Iron is a natural fertilizer and an essential nutrient for the synthesis of chlorophyll and electron transport proteins in plants, including phytoplankton. Without it, they are unable to reduce carbon dioxide and create the organic compounds needed for their survival (Paytan & Street, 2005). John Martin of the Moss Landing Marine Laboratories hypothesized that by increasing the amount of bio-available iron in oceans, overall photosynthetic processes would increase, and the ability for the ocean to sequester carbon from the atmosphere would increase accordingly; this was coined the Iron Hypothesis (Weier, 2001). This hypothesis has been tested on more than 10 different occasions and in all cases, massive phytoplankton blooms have resulted. However, the results are not consistent. Blooms persisted for variable periods of time under various, uncontrolled, open ocean conditions, and the effectiveness of phytoplankton blooms in sequestering carbon has yet to be confirmed (Paytan & Street, 2005). This minireview will explore the question pervading the minds of oceanographers, marine biologists/chemists, environmental scientists and geoengineers alike: can iron fertilization in the Southern Ocean, through increasing the primary productivity of phytoplankton, accelerate carbon sequestration in the deep ocean, therefore decreasing atmospheric CO2 concentrations? This review will examine papers on three iron fertilization experiments in the Southern Ocean and their findings. Themes between papers on each experiment will be examined, focusing first on the contributions of each paper to the progression of the field and then on weaknesses that could be addressed through further experimentation.
One of the original guiding principles upon which understanding of marine biogeochemistry was based was the correlation between macronutrient availability and phytoplankton production. Yet, observations and experiments conducted in the 1920s suggested high marine nutrient levels but low phytoplankton biomass (High Nutrient Low Chlorophyll or HNLC) occurring uniquely in the Southern Ocean. This dilemma came to be known as the Antarctic paradox (Broecker & Peng, 1991). It was the attempt to reconcile the Antarctic paradox that spurred research on nutrient levels in the Southern Ocean. It was not until the late 1980s that improvements in analytical techniques confirmed low dissolved iron levels and thus the potential to accelerate phytoplankton processes in the open Southern Ocean via iron fertilization (Ducklow, Hanson, Field, 2000). With the emergence of the Iron Hypothesis came the increased importance of iron’s role in the Southern Ocean, as the threat of dangerous atmospheric carbon levels loomed with no solution in sight. The study of the response of biogeochemical cycling in the surface mixed layer to iron enrichment was unfortunately limited at the time. Modelling simulations of the influence of iron enrichment in the Southern Ocean on atmospheric CO2 levels had yet to be verified and validated (Broecker & Peng, 1991). In the absence of data, such models assumed that iron enrichment resulted in complete utilization of upper ocean macronutrients. Yet these predictions did not align with results from lab experiments (Boyd et al., 2007) nor observations on natural blooms in the open Southern Ocean (Keith & Moore, 1999). These issues could only be addressed by conducting an in-situ iron fertilization experiment in open Southern Ocean waters to assess what controls the magnitude of phytoplankton stocks.
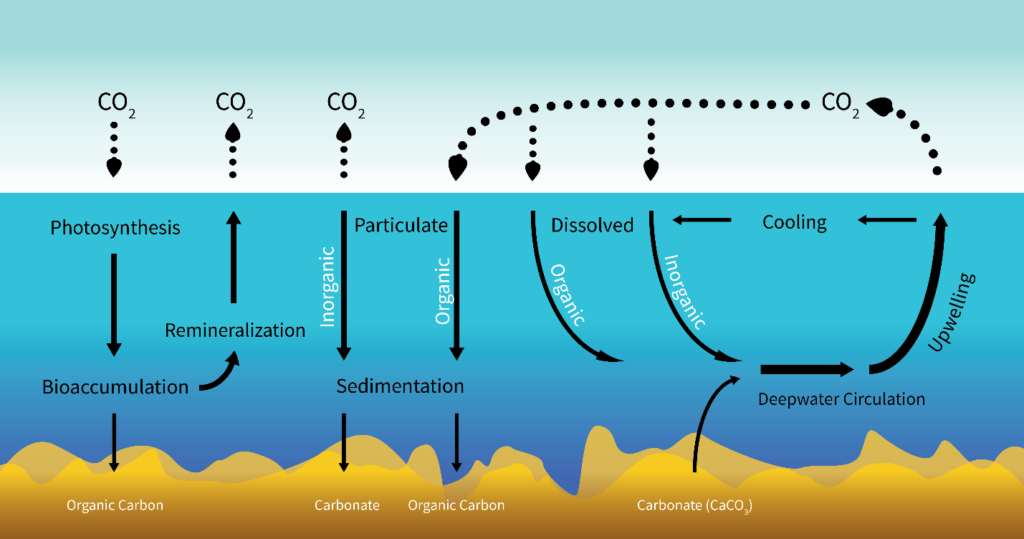
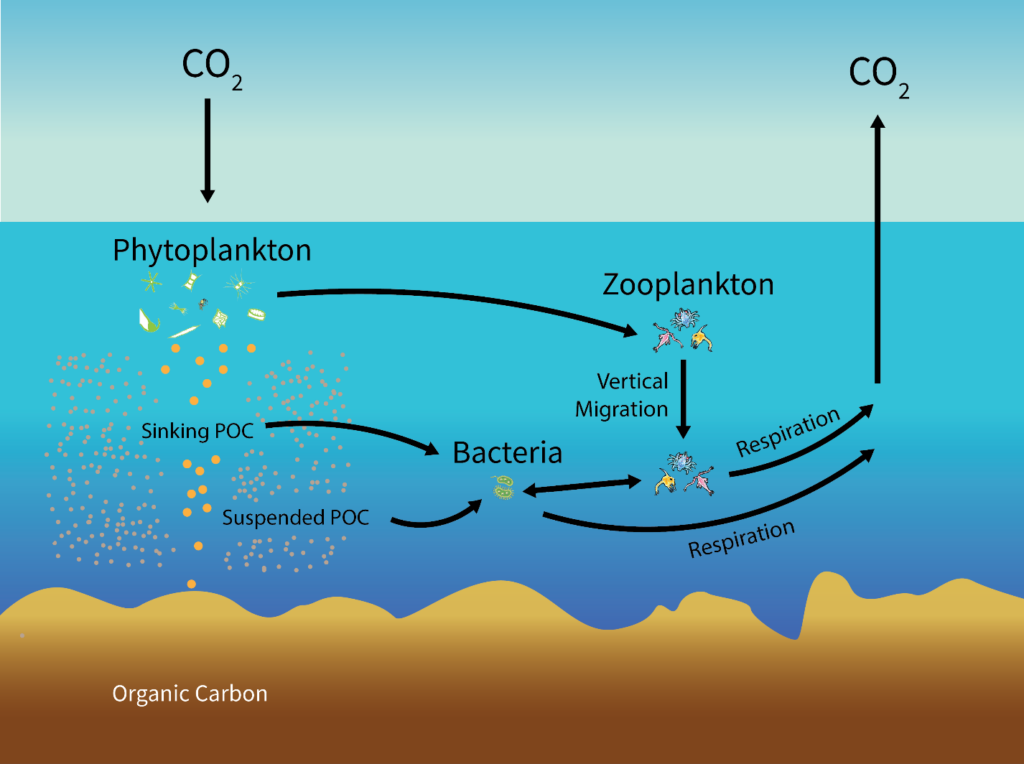
There are two steps to sequestering atmospheric carbon. First, removal from the atmosphere; second, conversion to a form that is unable to re-enter the atmosphere. Currently, the most effective carbon removal on the planet is performed by photosynthesizers, a role filled in ocean ecosystems by phytoplankton. Phytoplankton in the upper ocean fix carbon dioxide using solar energy. This fixation results in particulate organic carbon (POC), which is grazed upon by herbivorous zooplankton or consumed directly or indirectly by heterotrophic microbes feeding on solubilized remains of phytoplankton. Between 1 and 40% of the primary production is exported out of the upper ocean, and it exponentially attenuates towards the base of the mesopelagic zone at around 1,000m deep (Broecker & Peng, 1991). Remineralization of organic matter in the oceanic water column converts the organic carbon back to carbon dioxide. Hence, only about 1% of the surface production reaches the sea floor (Fig. 1). Carbon that reaches the sea floor to enter the lithosphere exits as inorganic calcium carbonate, which reacts with the low-pH ocean to produce CO2 to be released into the atmosphere, however, the majority remains sequestered. This cycle is called the biological pump, and it is one of several ways carbon can be sequestered in the ocean. Other oceanic carbon inputs include particle deposition, biotic debris, and atmospheric CO2 diffusion, and are sequestered by processes not specified in this paper (Fig. 2).
Literature review
SOIREE (Southern Ocean Iron Experiment): 1999
SOIREE was the first in-situ iron fertilization experiment undertaken in the polar waters of the Southern Ocean. Approximately 165 mol/km2 of FeSO4 · 7H2O was added to a 65-m deep surface mixed layer over an area of 50 km2. Croot et al. (2001) sought to distinguish SOIREE as an experiment that pointed to the previously unheralded importance of Fe(II) and organic complexation in the Southern Ocean. This mindset was shared amongst many of the marine biogeochemists studying SOIREE. Boyd et al. (2000) were convinced the findings of SOIREE not only provided context and significance for iron’s role in surface phytoplankton ecosystems, but allegedly confirmed the Iron Hypothesis in its findings on “the relative roles of iron supply, uptake, algal growth and community structure, and grazing” (p. 699). From further study on iron fertilization it is evident the researchers had not confirmed the Iron Hypothesis in its entirety, as both the mechanisms and rate or length of enhanced photosynthesis had yet to be determined. Additionally, the mechanism by which carbon dioxide was sequestered remained a mystery.
The advantage of in-situ experiments is the incorporation of natural large-scale ocean phenomena that potentially affect the rate, length, and efficiency of photosynthetic carbon sequestration absent from underdeveloped simulations or lab experiments. These phenomena could provide insight on the length of time carbon is sequestered away in the ocean before returning to the atmosphere as carbon dioxide (Fig. 2). Law & Boyd (2001) recognized this gap in the research in their conclusions, stating that the fate of accumulated carbon, once used in photosynthesis, could only be speculated. Whether it was remineralized into inorganic CO2 and returned to the atmosphere or subducted along with the carbon-rich remains of phytoplankton – or both – was undetermined. Thus, the longer-term sequestration of carbon in the Southern Ocean could not be extrapolated and the magnitude of the iron enrichment effect on marine production and atmospheric CO2 remained uncertain (Buesseler, 2004).
SOFeX (Southern Ocean Iron Experiment): 2002
Between SOIREE and SOFeX the German EisenEx (2000) iron fertilization experiment took place in the Southern Ocean but will not be discussed in this review due to its proximity to SOIREE (occurring less than a year after the first experiment). Consequently, the advancement in the field of marine biogeochemistry and insight on the Iron Hypothesis were minimal. One notable finding is the influence of mechanical stirring of the ocean surface on phytoplankton bloom success. EisenEx saw a bloom four times more efficient in drawing CO2 from the atmosphere than the SOIREE bloom due to stormier oceans in the northern section of the Southern Ocean, north of the Antarctic Polar Front (Bakker et al., 2005). Despite this finding, the researchers of EisenEx had made no progress on examining the biological or carbon systems that drive phytoplankton carbon sequestration.
SOFeX was the first iron fertilization experiment designed to examine the biological and carbon system response to the effects of purposeful addition of iron to high-nutrient low-chlorophyll (HNLC) waters in the Southern Ocean, specifically the polar front zone (PFZ). Multiple enrichments were performed on areas of 225 km2 to total approximately 88 mol/km2 of FeSO4 north and south of the PFZ (Bishop, 2004). In previous iron fertilization experiments in the Southern Ocean, the demonstration of an obvious carbon flux response in the atmosphere or the phytoplankton ecosystem was absent, although in every case enhanced primary production and a biomass increase was reported (Buesseler, 2004). SOFeX sought to eliminate the uncertainty surrounding the effectiveness of iron fertilization in sequestering CO2 by taking a multifaceted approach to measuring carbon flux in the ocean and atmosphere.
In marine environments, sequestration often proceeds via the biological pump, which incorporates phytoplankton photosynthesis, bioaccumulation, and particulate organic carbon (POC) sedimentation (Fig. 1) (Herndl & Reinthaler, 2013). This process is more specific than the cycling SOIREE researchers explored (Fig. 2) and directly addresses the mechanisms that constitute the relationship between phytoplankton and carbon sequestration. Researchers of the SOFeX experiment measured the extent of the biological pump in iron fertilized areas. Their approach would account for the gap Law & Boyd recognized in their research in 2001: the unknown fate of accumulated carbon once utilized in phytoplankton photosynthesis. The amount of carbon that sank to the ocean floor because of fertilization could finally be determined by updated technology not available during SOIREE. Bishop et al. (2004) deployed autonomous submarine sensors to investigate the systematics of POC export with an optically derived carbon flux index at depths of up to 1000 m. Buesseler et al. (2004) engaged an autonomous float to measure carbon flux at the ocean’s surface. By interpreting the data obtained from these instruments, SOFeX researchers concluded that iron addition indeed had a measurable impact on carbon sequestration rates. Carbon flux increases were not limited to the top, iron-fertilized layer of the ocean, but also below in the “shadow” of the SOFeX patch (Buesseler, 2004).
Researchers, however, admitted to difficulties in predicting the impact of iron fertilization on atmospheric carbon composition because the timespan of carbon remineralization and the large-scale impacts of iron enrichment on processes below the surface layer had yet to be understood. In other words, SOFeX demonstrated technological progress in terms of carbon inputs to a biological system but lacked the acknowledgement of carbon outputs. Despite prolonged observation of the bloom, the absence of data on the magnitude of remineralization left the potential for gaps when considering net sequestration over a long period of time. They “[did] not know whether the bloom eventually led to substantially higher C export after [they] left, or whether organic matter was remineralized within the surface ocean, resulting in no additional impact on C sequestration.” (Buesseler, 2004, p. 417). This had the potential to pose an issue for geoengineers exploring iron fertilization as an option for carbon capture.
Even excluding remineralization, many researchers who studied SOFeX are worried that the amount of carbon dioxide sequestered in experiments is not nearly enough to address rising atmospheric CO2 concentrations. Carbon flux measurements were taken for the first time during SOFeX, and their results were not promising. Coale et al. (2004) found the flux of carbon, although significant, was similar in magnitude to that of natural blooms in the Southern Ocean and thus small relative to global carbon budgets and proposed geoengineering plans to sequester atmospheric carbon dioxide in the deep sea. Buesseler et al. (2004) failed to see how iron fertilization with such a low C(sequestered): Fe(added) export efficiency could scale up to solve larger global C imbalance problems without consuming tons of iron salts. Only further exploration of carbon transport within and below phytoplankton blooms as well as further insight on the mechanisms of carbon uptake would provide geoengineers with the information needed to continue or abandon iron fertilization as an option for lowering atmospheric CO2 levels.
LOHAFEX (Iron Fertilization Experiment): 2007
LOHAFEX is the most recent iron fertilization performed in the Southern Ocean. ~101 mol/km2 of FeSO4 · 7H2O was added to an area of 150 km2. While all previous experiments showed an enhancement of primary production and biomass in response to iron increases in phytoplankton blooms, the relatively short duration of these studies did not allow researchers to determine the fate of exported POC from said blooms. Many studies on LOHAFEX aimed to understand the impact of an artificial iron-induced bloom in terms of mesopelagic remineralization processes (Martin et al., 2013). It is important to note that the shallow export flux of POC, often measured at 100 m, generally does not sequester carbon from the atmosphere for climatically relevant time scales. Long-term sequestration requires POC to sink below the permanent thermocline, and it is this deeper flux that would need enhancing for geoengineering to work (Buesseler, 2008). POC flux can decrease sharply between these two depths, and the magnitude of this decrease depends on the biological make-up in the surface and mesopelagic (Boyd & Buessler, 2009; Jacquet et al., 2008; Buesseler, 2007; Bishop & Lam, 2007). Thus, enhancing POC export does not necessarily enhance carbon sequestration. Therefore, examining both the remineralization processes and the depth to which particles sink, as demonstrated by LOHAFEX, is of the utmost importance when determining the sequestration potential of iron fertilization.
Advances in sediment collection and analysis allowed researchers to differentiate between types of POC export, revealing the majority of POC undergoing downward flux is faecal matter from zooplankton feeding on phytoplankton in excess. The decrease of faecal pellets with depth, and the increase in unrecognisable detritus particles observed points to intense reprocessing (consumption & excretion) of faecal pellets by heterotrophic bacteria and plankton at greater depths (Ebersbach et al., 2014). Ebersbach et al. believe this means two things, both of which result in an increase of carbon output from the system: i) the smaller, reprocessed faecal pellets sink more slowly, providing a greater chance for remineralization before descending past the thermocline, ii) heterotrophic bacteria in the process of consuming faecal pellets release CO2 as they respire and defecate. Despite this, mesopelagic remineralization levels during iron fertilization remain far below natural mesopelagic remineralization levels (Ebersbach et al., 2014). Jacquet et al. (2008) found that export from large, artificial phytoplankton blooms is less prone to remineralization than natural blooms. Their explanation for their observations is incompatible with Ebersbach’s analysis, for Jacquet assumes a faster transfer of matter through the water column leaving less time for mesopelagic remineralization to occur, even including heterotrophic reprocessing. Jacquet claims heterotrophic reprocessing is slowed because of low water temperatures. This conflict around the speed of POC sinking has yet to be resolved.
In addition to remineralization research, many studies on particle flux characterisation and sedimentation patterns within and below the phytoplankton bloom reveal particle flux decreases strongly between 100 and 200–450m (Martin et al., 2013). This indicates that little POC is attaining depths below the thermocline, indicating that long-term sequestration is unlikely to be enhanced. On top of this, Martin et al. (2013) claim iron fertilization enhances neither shallow export nor deep POC flux based on their observations, supporting Ebersbach’s analysis. These findings contradict the idea that iron fertilization stimulates POC export and sequestration in Southern Ocean HNLC conditions. However, such conclusions ignore the higher transfer efficiencies of flux to depths that have been reported upon collapse of these artificial blooms. With the deaths of so many organisms and their carbon carcasses falling to the ocean floor, large quantities of carbon sink past the thermocline due to sheer numbers. LOHAFEX was designed to be a long-term experiment for this very reason, allowing the iron-fertilized phytoplankton bloom to be monitored “until its senescence and decay, thereby closing a gap in our understanding of carbon transport” (Thiele et al., 2012). Unfortunately, the long-term monitoring of the LOHAFEX bloom never came to fruition due to its early conclusion in response to protests from environmental campaigns about the potentially harmful effects of iron fertilization.
Conclusion
The probability that iron fertilization will be implemented on a large scale to reduce atmospheric carbon dioxide is slim. The optimism arising from the initial conception of the Iron Hypothesis has faded, and which is supported by scientific evidence. As technology improves and datasets grow, our understanding of oceanic carbon sequestration has increased greatly, alongside the realization that oceanic carbon fixation processes are much more complicated than originally believed. These experiments are costly and difficult to run, and the environmental side effects of interfering with an ecosystem to such a great extent are unknown. It is for this reason that iron fertilization experiments in the Southern Ocean have not taken place in over a decade, and computers are taking over in the hopes that climate models will provide the solution to excessive atmospheric CO2 levels that the world is waiting for.
The real barrier to answering the outlined research question stems not from large gaps in knowledge or unclear results from research. It comes from the distrust of geoengineering. Scientists and the public alike fear repercussions from the deliberate imbalance of biological systems and nutrient cycles. This is not an unjustified fear, for it is the anthropogenic imbalance of global systems which generated dangerous climate change in the first place. Luckily, in-situ experiments are no longer the only method of accumulating accurate data, for models have improved drastically since 1999. Key findings from iron fertilization experiments offer new insights for modellers, although a limited number of these findings can be extrapolated directly to regional and seasonal scales for iron enrichment (Table 1). Such limited extrapolation speaks to limitations in experimental design and to uncertainties in the understanding of iron biogeochemistry (Boyd et al., 2007).
Modelling studies can improve our understanding of iron biogeochemistry by allowing for different conditions to be altered in a short time span and without risk of environmental repercussions. For example, as opposed to adding iron in one large dose, breaking the addition up into smaller, more frequent doses could result in more sustained blooms. Altering other nutrient concentrations such as silicon, barium, or nitrogen may be effective (Boyd et al., 2007). The technologies available today (and the countless more that will be available in the future) will allow research into iron fertilization as a method of accelerated carbon sequestration to be explored deeper than ever before. Perhaps one day iron will be more than an essential nutrient – it will be the key to maintaining life on Earth.
Acknowledgements
The author would like to acknowledge the support of Dr Emma Davy in writing this paper and for running an incredible class.
References
- Bakker, D. C. E., Bozec, Y., Nightingale, P. D., Goldson, L., Messias, M.-J., de Baar, H. J. W., … Watson, A. J. (2005). Iron and mixing affect biological carbon uptake in SOIREE and EisenEx, two Southern Ocean iron fertilisation experiments. Deep Sea Research Part I: Oceanographic Research Papers, 52(6), 1001–1019. https://doi.org/10.1016/j.dsr.2004.11.015
- Bishop, J. K. B. (2004). Robotic Observations of Enhanced Carbon Biomass and Export at 55 S During SOFeX. Science, 304(5669), 417–420. https://doi.org/10.1126/science.1087717
- Block, B. (2018, April 20). Oceans Absorb Less Carbon Dioxide as Marine Systems Change | Worldwatch Institute. Retrieved April 20, 2018, from http://www.worldwatch.org/node/6323
- Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., … Watson, A. J. (2007). Mesoscale Iron Enrichment Experiments 1993-2005: Synthesis and Future Directions. Science, 315(5812), 612–617. https://doi.org/10.1126/science.1131669
- Boyd, Philip W., Watson, A. J., Law, C. S., Abraham, E. R., Trull, T., Murdoch, R., … Zeldis, J. (2000). A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature, 407(6805), 695–702. https://doi.org/10.1038/35037500
- Boyd, P.W., & Law, C. S. (2001). The Southern Ocean Iron RElease Experiment (SOIREE)—introduction and summary. Deep Sea Research Part II: Topical Studies in Oceanography, 48(11–12), 2425–2438. https://doi.org/10.1016/S0967-0645(01)00002-9
- Buesseler, K. O. (2004). The Effects of Iron Fertilization on Carbon Sequestration in the Southern Ocean. Science, 304(5669), 414–417. https://doi.org/10.1126/science.1086895
- Buesseler, K. O., Doney, S. C., Karl, D. M., Boyd, P. W., Caldeira, K., Chai, F., … Watson, A. J. (2008). ENVIRONMENT: Ocean Iron Fertilization–Moving Forward in a Sea of Uncertainty. Science, 319(5860), 162–162. https://doi.org/10.1126/science.1154305
- Buesseler, K. O., Lamborg, C. H., Boyd, P. W., Lam, P. J., Trull, T. W., Bidigare, R. R., … Wilson, S. (2007). Revisiting Carbon Flux Through the Ocean’s Twilight Zone. Science, 316(5824), 567–570. https://doi.org/10.1126/science.1137959
- Buesseler Ken O., & Boyd Philip W. (2009). Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnology and Oceanography, 54(4), 1210–1232. https://doi.org/10.4319/lo.2009.54.4.1210
- Butler, A. (2005). Marine Siderophores and Microbial Iron Mobilization. BioMetals, 18(4), 369–374. https://doi.org/10.1007/s10534-005-3711-0
- Coale, K. H. (2004). Southern Ocean Iron Enrichment Experiment: Carbon Cycling in High- and Low-Si Waters. Science, 304(5669), 408–414. https://doi.org/10.1126/science.1089778
- Croot, Peter L., Bowie, A. R., Frew, R. D., Maldonado, M. T., Hall, J. A., Safi, K. A., … Law, C. S. (2001). Retention of dissolved iron and Fe(II) in an iron induced Southern Ocean phytoplankton bloom. Geophysical Research Letters, 28(18), 3425–3428. https://doi.org/10.1029/2001GL013023
- Ebersbach, F., Assmy, P., Martin, P., Schulz, I., Wolzenburg, S., & Nöthig, E.-M. (2014). Particle flux characterisation and sedimentation patterns of protistan plankton during the iron fertilisation experiment LOHAFEX in the Southern Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 89, 94–103. https://doi.org/10.1016/j.dsr.2014.04.007
- Hanson, R. B., Ducklow, H. W., & Field, J. G. (2000). The Changing Ocean Carbon Cycle: A Midterm Synthesis of the Joint Global Ocean Flux Study. Cambridge University Press. Herndl, G. J., & Reinthaler, T. (2013). Microbial control of the dark end of the biological pump. Nature Geoscience, 6(9), 718–724. https://doi.org/10.1038/ngeo1921
- Jacquet, S. H. M., Savoye, N., Dehairs, F., Strass, V. H., & Cardinal, D. (2008). Mesopelagic carbon remineralization during the European Iron Fertilization Experiment. Global Biogeochemical Cycles, 22(1). https://doi.org/10.1029/2006GB002902
- Lam, P. J., & Bishop, J. K. B. (2007). High biomass, low export regimes in the Southern Ocean. Deep Sea Research Part II: Topical Studies in Oceanography, 54(5), 601–638. https://doi.org/10.1016/j.dsr2.2007.01.013
- Lenntech. (n.d.). Iron (Fe) and water. Retrieved April 20, 2018, from https://www.lenntech.com/periodic/water/iron/iron-and-water.htm
- Lin, I., Liu, W. T., Wu, C.-C., Wong, G. T. F., Hu, C., Chen, Z., … Liu, K.-K. (2003). New evidence for enhanced ocean primary production triggered by tropical cyclone. Geophysical Research Letters, 30(13). https://doi.org/10.1029/2003GL017141
- Martin, P., van der Loeff, M. R., Cassar, N., Vandromme, P., d’Ovidio, F., Stemmann, L., … Naqvi, S. W. A. (2013). Iron fertilization enhanced net community production but not downward particle flux during the Southern Ocean iron fertilization experiment LOHAFEX: NCP AND PARTICLE FLUX DURING LOHAFEX. Global Biogeochemical Cycles, 27(3), 871–881. https://doi.org/10.1002/gbc.20077
- Moore J. Keith, Abbott Mark R., Richman James G., Smith Walker O., Cowles Timothy J., Coale Kenneth H., … Barber Richard T. (1999). SeaWiFS satellite ocean color data from the Southern Ocean. Geophysical Research Letters, 26(10), 1465–1468. https://doi.org/10.1029/1999GL900242
- Peng, T.-H., & Broecker, W. S. (n.d.). Dynamical limitations on the Antarctic iron fertilization strategy | Nature. Retrieved May 2, 2018, from https://www.nature.com/articles/349227a0
- Sandy, M., & Butler, A. (2009). Microbial Iron Acquisition: Marine and Terrestrial Siderophores. Chemical Reviews, 109(10), 4580–4595. https://doi.org/10.1021/cr9002787
- Sillén, L. G., Martell, A. E., & Bjerrum, J. (1964). Stability constants of metal-ion complexes. 2(17). Chemical Society.
- Street, J. H., & Paytan, A. (2005). Iron, phytoplankton growth, and the carbon cycle. Metal Ions in Biological Systems, 43, 153–193.
- The Earth Institute. (2009, November 18). Oceans’ Uptake of Manmade Carbon May Be Slowing – The Earth Institute – Columbia University. Retrieved April 20, 2018, from http://www.earth.columbia.edu/articles/view/2586.
- Thiele, S., Fuchs, B. M., Ramaiah, N., & Amann, R. (2012). Microbial Community Response during the Iron Fertilization Experiment LOHAFEX. Applied and Environmental Microbiology, 78(24), 8803–8812. https://doi.org/10.1128/AEM.01814-12
- Weier, J. (2001, July 10). John Martin : Feature Articles [Text.Article]. Retrieved May 2, 2018, from https://earthobservatory.nasa.gov/Features/Martin/martin_4.php.
