Abstract
Recent studies have suggested that adolescent cancer may have better survival outcomes when treated at paediatric centres, which better emphasize enrolment in clinical trials and have more capacity to support the social and emotional needs of adolescents. This study investigated 616 cancer cases in adolescents aged 15-18 from 1995 to 2010 in British Columbia, Canada with data from the Childhood, Adolescent, and Young Adult Cancer Survivors Research (CAYACS) Program of the BC Cancer Research Agency (BCCA). This study examined whether referrals to the adult centres BC Children’s Hospital (BCCH) or BCCA were influenced by age, socioeconomic status, rurality, seasonality, radiotherapy treatment, different diagnoses based on the International Classification of Childhood Cancer (ICCC), and driving time to each centre. Between 1995 and 2010, only 27% of adolescent cancer patients in BC were referred to the BCCH, the only paediatric oncology centre in BC. Rural patients might have limited accessibility to BCCH, despite referral, due to travel restrictions and costs. As a result, patients are less likely to be referred to their closest cancer treatment centre as driving time increases (OR 0.995, P= 1.9e-10). Odds ratios of each modifier to BCCA or BCCH were calculated using univariate and multivariate logistic regression models in R Studio 3.5.1. Overall, 80% of younger adolescent (age 15-16) were referred to BCCH and only 14% of older adolescents (age 17-18) were referred to BCCH, which suggested that older adolescents were less likely than younger adolescents to be referred to BCCH (P= <2e-16). Additionally, leukaemia and Central Nervous System (CNS) cancer patients were more likely than all other patients to be referred to BCCH (P= 0.0014). The study of referral patterns is an essential factor when determining adolescent cancer survival rate.
Introduction
On average, 412 adolescents in Canada aged 15-19 are diagnosed with cancer every year, and 73 die as a result (Canadian Cancer Registry, 2011). Over the past 50 years, the 5-year survival rate for childhood cancer (ages 0-14) has significantly increased by 40% in the United States due to improved therapy and supportive care. Nevertheless, the same progress is not seen for adolescent patients (ages 15-19). This is the “Adolescent and Young Adult Cancer Survival Gap”, which is observed in the US and Canada and can be attributed to a combination of adolescent cancer biology, varying responses to treatment, and a lack of enrolment in clinical trials (Bleyer, 2005; Pollock, 2007). The site of adolescent cancer treatment matters because paediatric centres often place greater emphasis on clinical trial participation and provide greater psychosocial support for adolescents, which can ultimately lead to better survival outcomes (Pollock, 2007; Brand et al., 2016). In British Columbia, the BC Children’s Hospital (BCCH) is the only children’s oncology treatment centre; therefore, the first aim of this study investigates where adolescents with cancer are receiving their treatment and what factors influence those referral patterns that ensure best treatment practices.
More specifically to BC, adolescent patients living in the northern and southern interior may experience greater constraints in accessing care due to longer travel times to BCCH and the scarcity of care providers. Travel impedance has been known to be a controlling factor in the utilization of health care systems (Kanaroglou et al., 2016). Thus, the second aim of this study investigates whether driving time influences referral decisions for adolescent cancer patients in BC. Such an approach encompasses the perspective of health geography, where patients’ locations can impede access to cancer treatment centres. Currently, there is no specialized adolescent cancer treatment unit nor universal guidelines for adolescent treatment at either paediatric or adult cancer centres in Canada or the US (Albritton & Bleyer, 2003).
This study analyzed data from 1995 to 2010 because the patient data up to 2010 was the most complete, comprehensive, and readily available. Despite the data being more than a decade old, the study on adolescent cancer patients’ accessibility is one of the first in British Columbia. Moreover, the “Adolescent and Young Adult Cancer Survival Gap” still occurs across Canada and there still lacks systematic guidelines on how adolescent cancer patients should be referred and medical subspecialties that specifically treat adolescent cancer patients.
Methods
Data source
The patient data was collected from the Childhood, Adolescent, and Young Adult Cancer Survivors Research Program (CAYACS) by the BC Cancer Research Agency. The CAYACS program is a longitudinal cohort study that follows children and adolescent cancer patients from their initial diagnoses (McBride et al., 2010). Consent for personal data was given by the BC Cancer Research Agency via a confidentiality agreement. This dataset included all individuals diagnosed with cancer (excluding non-melanoma skin cancer) between ages 15-18 inclusive (n= 616) from 1995 to 2010, who are BC residents at the time of diagnosis and survived at least one month from the diagnosis.
For this study, all BCCA centres were defined as adult centres (Vancouver Centre, Vancouver Island Centre, Fraser Valley Centre, Southern Interior Centre, Abbotsford Centre) while BCCH was the paediatric treatment centre. Between 1995 and 2010, the Southern Interior Centre was opened 3 April 1998 and the Abbotsford Centre was opened 25 August 2008. The Northern Centre was excluded from this study because it was opened in 2012. Therefore, the patient dataset was categorized as 1 January 1995 to 2 April 1998; 3 April 1998 to 24 August 2008; and 25 August 2008 to 31 December 2010.
Geocoding
The hospital locations were geocoded using the “Geocode Addresses Tool” in ArcGIS and the address locator was provided by the DMTI Route Logistic package. The DMTI Postal Code Suite contained polygon files of local delivery units (LDU) in BC that consist of 6-digit postal codes. Patient data was geocoded to the corresponding LDU.
Road network dataset
The road network dataset was created using the Network Analyst extension in ArcGIS from the DMTI Route Logistics package as a necessary procedure to produce driving times.
Driving time
Driving time of each patient to the closest cancer treatment centres was calculated using the OD cost matrix from the ArcGIS Network Analyst extension. In the driving time output, every patient had driving times to every centre calculated and ranked from shortest to longest. The shortest driving time to any centre indicated the closest facility for that patient.
Driving map
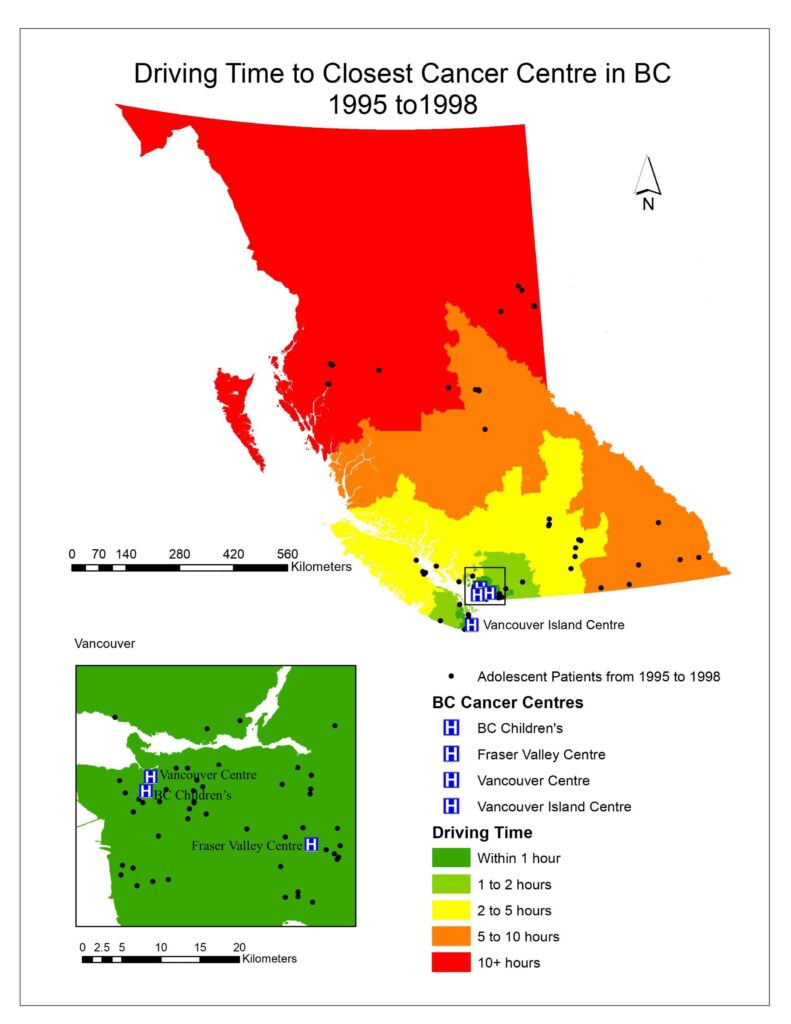
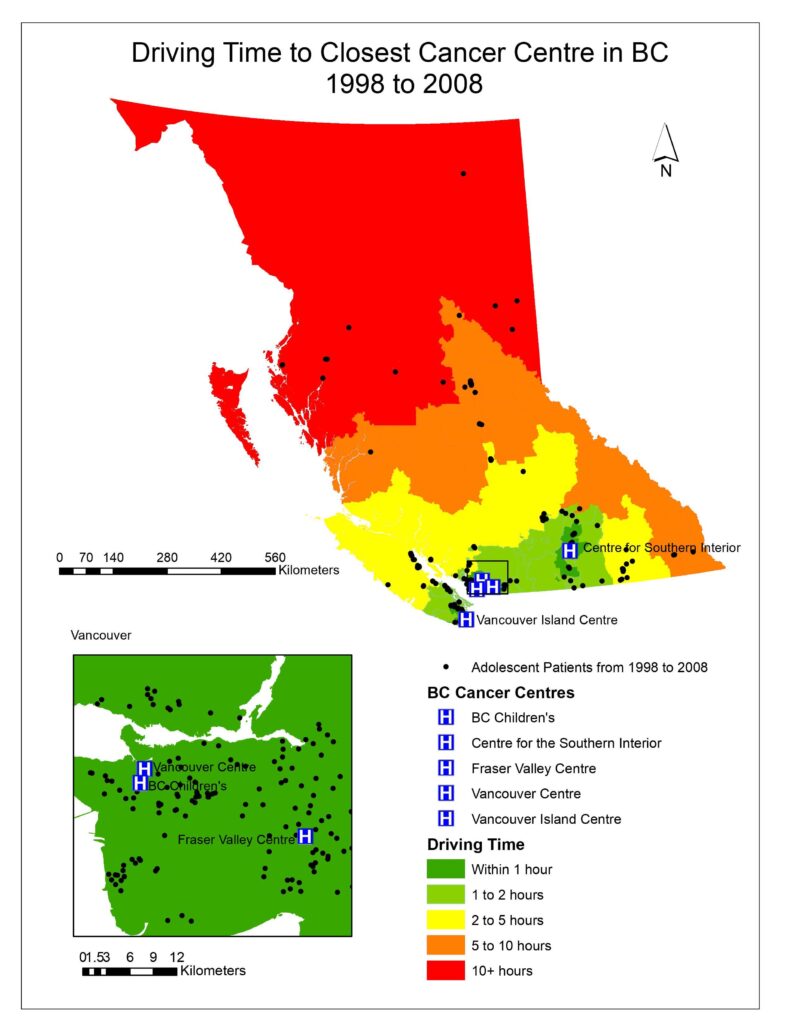
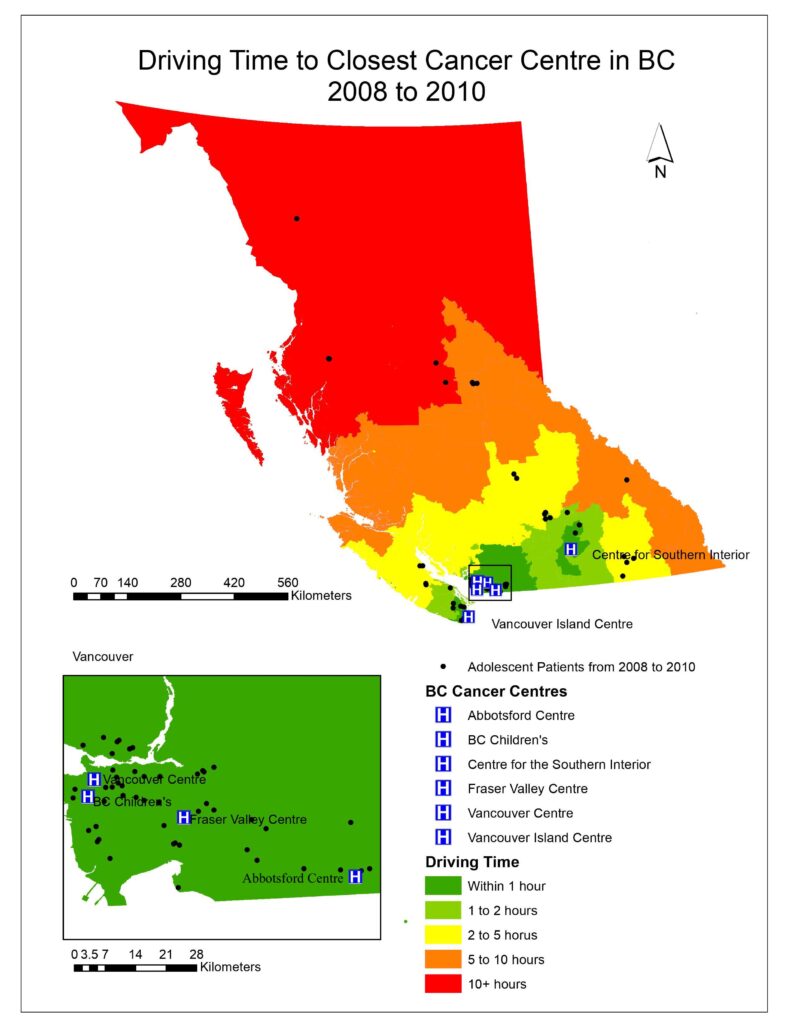
To visualize the driving times to the closest cancer treatment centre for patients in BC, aggregated dissemination areas (ADA) from Statistics Canada were used as the patient data was too small to represent the driving time for the entire province and spatial interpolation would not be accurate. After driving times were calculated for each ADA; driving time was categorized as falling within 1 hour, 1-2 hours, 2-5 hours, 5-10 hours, or 10+ hours (Figs. 1, 2, 3).
Statistical analysis
Univariate and multivariate logistic regressions between each modifier such as age, socioeconomic status (SES), ICCC categories, radiotherapy treatment, seasonality, rurality. Patients farther from paediatric centers were tested for whether they were referred to BCCH or BCCA on R Studio version 3.5.1., where odds ratios were calculated.
Results
Population referral characteristics
Between 1995 and 2010, a total of 616 adolescents between ages 15-18 were diagnosed with cancer in BC. Table 1 highlights the distribution of referral to every cancer treatment centre in BC based on age, income, rurality, regional health authority catchment, seasonality, and diagnosis groups. Overall, 59% of patients were referred to BCCA and 27% were referred to BCCH. Among BCCA referrals, 30% of patients were referred to the Vancouver centre and only 6% were referred to the Southern Interior centre.
The univariate logistics regression models (Table 2) showed that only age and diagnosis groups including leukaemia, CNS, germ cell, and thyroid carcinoma had a statistically significant impact on referral. 63% of older adolescents were referred to BCCA while 80% of younger adolescents were referred to BCCH. This showed that patients between ages 17-18 were less likely than patients between ages 15-16 to be referred to paediatric centres (OR 0.14, P= <2e-16) which is the most expected result similar to previous studies (Table 2).
Although referrals to BCCA and BCCH seem to be evenly distributed between ICCC diagnosis, leukaemia and CNS patients were more likely than all other patients to be referred to BCCH (OR 2.23, P=0.0013; OR 2.42, P= 0.0014). Germ cell and thyroid carcinoma are both less likely than all other diagnoses to be referred to BCCH (OR 0.39, P= 0.0029; OR 0.27, P= 0.0348). Sarcoma patients were more likely to be referred to BCCH but not with statistical significance whereas lymphoma patients were less likely to be referred to BCCH based on the odds ratio (Table 2).
Patients of higher SES, urban locality, and who were closer to BCCH were more likely to be referred to BCCH but not with statistical significance. The overall referral characteristics showed that referral based on neighbourhood income quintile was evenly distributed across all centres. Referral to the Southern Interior centre consisted of 54% urban and 46% non-urban patients whereas referral to all other centres consisted of mostly urban patients. Additionally, whether a patient received radiotherapy treatment did not affect whether a patient was treated at BCCH.
The multivariate logistic regression model tested for the combined effect of all the modifiers on referral to BCCA or BCCH (Table 3). The model showed that age was the only factor that impacted referral to BCCA or BCCH after all the factors were accounted for (OR 0.13, P < 2e-16).
Driving time and referral
Fig. 1, 2, 3 visualized the overall distribution of driving time to the closest cancer centre across BC. From 1995 to 1998, the driving time was shortest for patients living the Vancouver lower mainland where which was within 1 hour of their closest treatment centre, gradually increasing as one moves away to other parts of BC. This is expected as there were three treatment centres (Vancouver centre, Fraser Valley centre and BC Children’s) in the area, easing access to those three facilities for patients living there, translating to shorter driving times. Since the opening of the Southern Interior centre in 1998, driving time for patients in that area significantly decreased as indicated by the dark green cluster in Fig. 2. Nonetheless, driving time for patients in all the other parts of BC remained relatively the same. After the Abbotsford centre opened in 2008, the dark green region expanded further north and east from the Vancouver lower mainland, which shows that more patients had access to a cancer centre within 1 hour of driving time (Fig. 3). However, minimal change was observed in other parts of BC since the Abbotsford centre is still within the Fraser Valley, and often acts as an overflow centre to complement the Fraser Valley Centre in Surrey. The opening of the Southern Interior and Abbotsford centre shows that access to cancer treatment centre improved only locally. Between 1995 and 2010, 61% of patients were not referred to their closest treatment centre and only 35% were. As driving time increased, the patient’s referred centre was less likely to be their closest treatment centre (OR 0.995, P= 1.9e-10).
Discussion
Since older adolescents were less likely than younger adolescents to be referred to BCCH, age was one of the most important factors influencing referral which showed that older adolescents still had limited access to BCCH. This result is consistent with the lower referral rate to paediatric centres for patients aged 18-19 compared to those aged 15-17 according to a 2004 study by the Canadian Childhood Cancer Surveillance and Control Program (Klein-Geltink et al., 2004). To illustrate the effect of age on referral, the multivariate logistic regression also demonstrated that age was the only factor that had a statistically significant impact on referral when all the tested factors were combined.
Similarly, studies in North Carolina, Washington State, Georgia and Ohio found that referral to paediatric centres significantly decreased as adolescent age increases from 15 to 19 (Gordon et al., 2018; Howell et al., 2007; Yeager, et al., 2006). The contributing factor could be the physician conducting the referral: for example, although there is no defined age limit for adolescents to be treated at BCCH, there might still be a “perceived age limit” by the physician even though it might be beneficial for patients in their early 20s to be referred to paediatric centres (Gordon et al., 2018). Moreover, paediatric primary care physicians (PCP) were more likely to refer their patients to paediatric centres if they had colleagues that they personally knew working in these centres (Gordon et al., 2018).
Older adolescents living in rural areas of BC might prefer a BCCA centre because it is geographically closer and they can drive themselves to the treatment sessions despite the more specialized care provided in BCCH (Albritton et al., 2007). Older adolescent patients might also be reluctant to receive treatment at BCCH because they do not want to be perceived as children and want to be more connected to their peers who are also entering adulthood (Albritton et al., 2007; Howell et al., 2017; Klein-Geltink et al., 2005). The result showed that as driving time increased, adolescent patients were less likely to be referred to their closest centre, which can benefit rural patients. Since the driving time to their closest BCCA might be just as far as BCCH, families might choose BCCH instead due to better treatment options. In general, improved survival outcomes were seen at the paediatric centre because they provided supportive care more tailored towards the emotional needs of adolescents and inpatient care which improves survival outcomes in cases of adverse complications (Howell et al., 2007). More importantly, lack of access to BCCH for these older adolescents might suggest lack of enrolment in clinical trials, which may significantly impact their survival outcome (Parsons et al., 2015).
Referral was also influenced by diagnosis. Leukaemia (all subgroups) and CNS tumour patients were more likely than patients with other cancers to be referred to BCCH. According to the Surveillance, Epidemiology, and End Results (SEER) Program, 68.2% of average annual incidence per million of leukaemia from 1975-2000 occurred in children less than 5 years old (Bleyer et al., 2006). Similarly, higher incidence of CNS tumours was seen in children under 5 (33.7%) compared to adolescents between ages 15-19 (19.2%). This suggests that adolescent patients with cancer more commonly seen in younger children are more likely to be referred to BCCH. This is important when considering the survival outcome of referrals because the 5-year survival rate for leukaemia is often lower when it is not treated at paediatric centres (Howell et al., 2007).
Patients diagnosed with germ cell and thyroid carcinoma were less likely to be referred to BCCH and both cancers were more common in the older teens than in younger children according to the SEER Program (Bleyer et al., 2006). Those patients diagnosed with germ cell and thyroid carcinoma may also be seen by physicians with surgical specialties, which decreases their chances of being referred to BCCH (Albritton et al., 2007; Parsons et al., 2015).
Other ICCC diagnoses also affect referral but not with statistical significance. Bone tumours and soft tissue sarcomas are more likely to be treated at a paediatric centre where survival for patients with those cancers is usually favored (Howell et al., 2007). Lymphoma patients are less likely to be referred to BCCH, which is surprising because lymphoma is usually considered a more “paediatric-specific cancer” (Bleyer et al., 2006). This suggests a potentially unfavourable survival outcome for those lymphoma patients treated at BCCA, since survival may improve when they are treated at BCCH instead (Parsons et al., 2015). Furthermore, SES, rurality, seasonality, and distance to BCCH, which are internally correlated factors, did not influence referral with statistical significance. Since lower SES and rural patients are expected to have less accessibility to BCCH, the logistic regression showed that referral patterns were in fact not as affected by those factors than by age and diagnosis. This reflects health care access equality in British Columbia under the premise of this study.
Of greater implication for referral location is the patient’s potential survival rate. Each category of adolescent cancer has biologically distinct characteristics and thus requires careful consideration of whether an adult or paediatric center will be better suited for a particular patient (Wolfson et al., 2014). There are major benefits when treated is received at a paediatric centre, such as participation in clinical trials, peer support, and better follow-up care (De et al., 2011). Given the uniqueness of adolescent cancer, referral decisions are often complex and there is no “one size fits all” solution.
Conclusions
This study suggests that patients who were younger, of higher SES, urban, suffered from lymphoma, sarcoma, without radiotherapy, not traveling in the winter, and located farther from an adult centre would be more likely to be referred to BCCH. More specifically, age and diagnosis were the two major factors affecting adolescent cancer patient referrals in British Columbia. From a physician’s perspective, the awareness that older adolescents might be better treated at paediatric centres is critical (Yeager et al., 2006). Additional systematic efforts should also be implemented such as developing hospital policies that facilitate the appropriate referral based on each adolescent’s age and diagnosis, and improving access to these appropriately referred treatment centres to further reduce the AYA cancer gap (Gordon et al., 2018; Parsons et al., 2015). Currently, the Adolescent and Young Adult Committee has prioritized the identification of new treatment protocols tailored towards treating adolescent cancer, improving psychosocial support during therapy, and increasing the participation in clinical trials (Pollock, 2007).
It should be noted that referral location may differ from where patients actually receive treatment, because such data is not available through the CAYACS program. Nonetheless, this study contributed insights on access to adolescent cancer care in BC, with the additional dimension of driving time. This can also be considered in the planning of another paediatric hospital or cancer centre in other parts of BC, while further informing paediatric and adult oncologists about referral and survival patterns. Since the Northern BC Centre was omitted in this study due to the limited dataset timeline, this project should be repeated when data after 2012 becomes available. Furthermore, the two studies could be compared to analyze whether the Northern centre increased accessibility to care and survival for adolescents living in Northern BC. As a critical next step to this study, the 5-year survival outcome for this cohort and enrolment in clinical trials based on referral difference should also be examined.
Acknowledgements
The author would like to thank Dr Martin Putterman “for introducing me to Mary McBride, the program leader of CAYACS, who made this entire project possible”, Mary McBride and Dr Meera Rayar who “came up with the research topic and objectives which I was able to investigate”, Dr Brian Klingenberg “for supervising this directed study”, Dr. Trevor Dummer “for helping with the study design and consultation”, and Yang Zhang and Terry Tang who “have offered tremendous help in the CAYAC data organization”.
References
- Albritton, K., & Bleyer, W. A. (2003, December). The management of cancer in the older adolescent. Retrieved April 3, 2019, from https://www.ncbi.nlm.nih.gov/pubmed/14642921
- Albritton, K. H., Wiggins, C. H., Nelson, H. E., & Weeks, J. C. (2007). Site of oncologic specialty care for older adolescents in utah. Journal of Clinical Oncology, 25(29), 4616-4621. doi:10.1200/JCO.2006.08.4103
- Bleyer, A. (2005). The adolescent and young adult gap in cancer care and outcome. Current Problems in Paediatric and Adolescent Health Care, 35(5), 182-217. doi:10.1016/j.cppeds.2005.02.001
- Bleyer, A, O’Leary, M, Barr, R, Ries, L.A.G. (2006). Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. National Cancer Institute, NIH Pub. No. 06-5767. Bethesda, MD 2006.
- Brand, S. R., Pickard, L., Mack, J. W., & Berry, L. L. (2016). What Adult Cancer Care Can Learn From Paediatrics. Journal of oncology practice, 12(9), 765–767. doi:10.1200/JOP.2016.015057
- De, P., Ellison, L. F., Barr, R. D., Semenciw, R., Marrett, L. D., Weir, H. K., . . . for the Steering Committee for Canadian Cancer Statistics. (2011). Canadian adolescents and young adults with cancer: Opportunity to improve coordination and level of care. Canadian Medical Association Journal (CMAJ), 183(3), E187-E194. doi:10.1503/cmaj.100800
- Gordon, L. M., Johnson, R. H., Au, M. A., Langer, S. L., & Albritton, K. H. (2018). Primary care physicians’ decision making regarding initial oncology referral for adolescents and young adults with cancer. Journal of Adolescent Health, 62(2), 176-183. doi:10.1016/j.jadohealth.2017.09.006
- Howell, D. L., Ward, K. C., Austin, H. D., Young, J. L., & Woods, W. G. (2007). Access to paediatric cancer care by age, race, and diagnosis, and outcomes of cancer treatment in paediatric and adolescent patients in the state of georgia. Journal of Clinical Oncology, 25(29), 4610-4615. doi:10.1200/JCO.2006.07.6992
- Kanaroglou, P., Delmelle, E., Páez, A., & Taylor & Francis eBooks A-Z. (2015;2016;). Spatial analysis in health geography (New ed.). Farnham, Surrey, UK, England; Burlington, VT, USA;: Ashgate. doi:10.4324/9781315610252
- Klein-Geltink, J., Shaw, A. K., Morrison, H. I., Barr, R. D., & Greenberg, M. L. (2005). Use of paediatric versus adult oncology treatment centres by adolescents 15–19 years old: The canadian childhood cancer surveillance and control program. European Journal of Cancer, 41(3), 404-410. doi:10.1016/j.ejca.2004.10.023
- McBride, M. L., Rogers, P. C., Sheps, S. B., Glickman, V., Broemeling, A., Goddard, K., . . . Xie, L. (2010). Childhood, adolescent, and young adult cancer survivors research program of british columbia: Objectives, study design, and cohort characteristics. Paediatric Blood & Cancer, 55(2), 324-330. doi:10.1002/pbc.22476
- Parsons, H. M., Harlan, L. C., Schmidt, S., Keegan, T. H. M., Lynch, C. F., Kent, E. E., . . . the AYA
- Pollock, B. H. (2007). Where adolescents and young adults with cancer receive their care: Does it matter? Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 25(29), 4522-4523. doi:10.1200/JCO.2007.12.1715
- Statistics Canada. (2018, March 12). 2010 Census Boundary files. Retrieved February 3, 2019, from https://www12.statcan.gc.ca/census-recensement/2011/geo/bound-limit/bound-limit-2016-eng.cfm
- Statistics Canada. (2019, January 28). Canadian Cancer Registry (CCR). Retrieved February 2, 2019, from http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207
- Wolfson, J., Sun, C., Kang, T., Wyatt, L., D’Appuzzo, M., & Bhatia, S. (2014). Impact of treatment site in adolescents and young adults with central nervous system tumors. JNCI : Journal of the National Cancer Institute, 106(8), dju166-dju166. doi:10.1093/jnci/dju166
- Yeager, N. D., Hoshaw-Woodard, S., Ruymann, F. B., & Termuhlen, A. (2006). Patterns of care among adolescents with malignancy in ohio. Journal of Paediatric hematology/oncology, 28(1), 17.
