Abstract
Clozapine is a crucial antipsychotic medication to treat schizophrenia and has shown valuable benefits in doing so. However, there can be rare, but potentially fatal adverse drug reactions to the medication. Therefore, it is essential that there is careful monitoring of the patient while on the medication. A retrospective study was conducted to examine the effects of clozapine on vitals during the first 2 days of initiation. There were 43 (29 male, 16 female) charts analyzed retrospectively, and the average age of the patients was 45 years old. The average dose of clozapine on the first day was 9.3mg and 15.7mg on the second day. Regarding vitals, the temperature peaked 5 hours after administrating clozapine on day 1 and day 2. Furthermore, the respiration rate peaked 4 hours post-dose on the second day and the heart rate increased the most on the second day indicating a dose-related response. There was no trend in blood pressure changes that can be inferred from the data; however, individual variations in blood pressure fluctuated considerably. The study was limited due to a lack of data in patient charts, and it may be of interest to do a prospective study to gain more insight.
Introduction
Clozapine is a unique, atypical antipsychotic medication that is effective for treating the positive and negative symptoms of schizophrenia (McEvoy et al., 2006). However, it may cause severe side effects such as agranulocytosis, seizures, cardiovascular/respiratory arrest and myocarditis (Merrill et al. 2006; Miller 2000). In fact, approximately 8.1% of patients are taken off therapy due to a serious side effect (Grohmann, 1989). There are significant side effects with treatment, and it is essential to treat schizophrenia effectively as it is a severe mental disorder that can cause considerable disability such as delusions, hallucinations, problems with concentration, and a lack of motivation among other debilitating aspects.
This study aimed to determine the effects of clozapine on patient’s vitals during the first two days of dosage titration and if there is an appropriate amount of monitoring while at a community initiation of therapy. Currently at the Fraser Health Psychosis Treatment Optimizing program (PTOP), patients starting clozapine have their blood pressure (standing and lying), temperature, pulse, and respiration measured before dosage administration, 15 minutes, one hour, two hours, three hours, four hours, five hours and six hours post-dose for the first two days of therapy (Fraser Health Authority, 2015). Such intense monitoring can make it difficult for providers to follow and increases the amount of laborious monitoring towards the patient.
Although there are significant and life-threatening side effects associated with clozapine, there are no evidence-based monitoring plans (Canadian Agency for Drugs and Technologies in Health, 2010). Furthermore, monitoring guidelines across the world were examined to determine if a trend could be found. The Commonwealth regions (New Zealand, Australia, Wales, Manchester) and California were investigated and found no consistent monitoring patterns between the regions.
When the health authorities were contacted for the rationale behind the guidelines they described it as being based on drug monographs and Maudsley’s prescribing guidelines (11th edition). When examining the Maudsley’s recommendation, it suggests to either give the first dose in the morning and monitor for 3 hours or give the first dose in the evening before retiring to avoid monitoring (Taylor et al., 2015). The drug monograph recommendations are based on an expert panel review board and are very similar to Maudsley’s monitoring proposals (O’Brien 2004, and. Novartis Pharmaceuticals, 2003). Thus, the current drug monitoring guidelines within the Fraser Health Authority significantly err on the safer side when compared to other literature sources of clozapine monitoring.
Methods
Between the period of 2012-2015, a retrospective review of PTOP clozapine therapy initiation within the Fraser Health was examined for vital sign data (blood pressure, respiration rate, heart rate and temperature). The study searched for any changes in blood pressure and heart rate at each of the current monitoring intervals; the monitoring levels are attained at baseline 15 minutes, one hour, two hours, three hours, four hours, five hours and six hours post-dose for the first two days of therapy). Furthermore, demographic information for confounding was also examined such as medication, age, comorbidities, sex, prior exposure to clozapine, blood work, and ECG monitoring.
All data were examined for means, standard deviations, and missing information. Furthermore, data collection was analysed for consistency in recording of data, clinician accuracy and discrepancies. In addition, bias was also examined such as in areas of selection, design, response, reporting, and measurement. Starting dose clozapine was also evaluated. The primary objective of this study was to determine any changes in vital signs associated with clozapine initiation in the first two days and the time of peak effect. The secondary goal was to determine if there are patient factors (e.g. age, gender, ethnicity, number or type of concurrent medications) that are associated with any observed changes in vital signs.
Results
Demographics
There were 174 patients made up PTOP, but n = 131 were excluded, leaving 43 charts to examine in this study. The patients were excluded due to a lack of patient monitoring information. There were 29 male and 16 female patients, and the average age was 45 years old. The mean dose on the first day was 9.3mg and the average treatment dose on the second day was 15.7mg. All available past substance and medication history were collected (Table 1 and 2). Table 3 shows pre-existing risk factors and comorbidities of the patient. It is important to note that due to the nature of data collection Tables 1, 2, and 3 are anticipated to be incomplete (retrospective study).
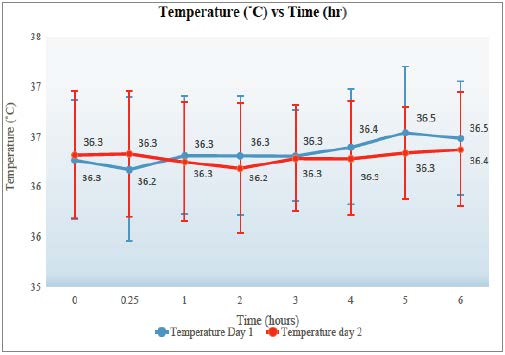
Temperature
Temperature peaked at 5 hours post-dose for both days 1 and 2. The maximum temperature for a patient was 37.6˚C, and the minimum was 34.1˚C with a standard deviation of 0.60. Refer to Figure 1 for the graph of temperature.
Respiration
The maximum respiration rate recorded was 30 breaths/minute, and the lowest was 10 breaths/minute. The standard deviation is 2.5. The respiration for Day 2 spikes at the 4th hour, 19 breaths/minute, and appears slightly higher than the first day. Refer to Figure 2.

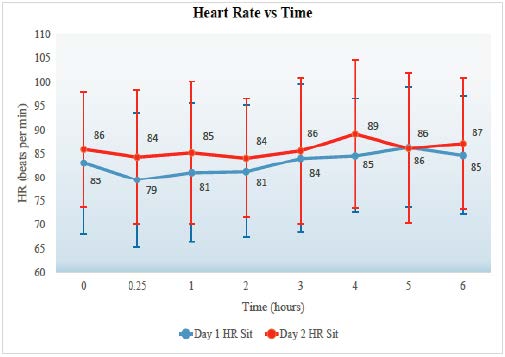
Heart rate
Figure 3 shows the maximum heart rate was 121 beats per minute (bpm) and the minimum was 49 bpm. The lying heart rate standard deviation for the patient population is 13.9 bpm. The peak in pulse appears to occur between hours 3.5-5.5. The second day seems to have a more significant increase in heart rate compared to the first day. Figure 4 shows the maximum average heart rate change from lying to standing and occurs at hour 3 in Day 1, 17.1 bpm, and the lowest average change occurs at hour 2 in Day 1 (8.4). The standard deviation of the averaged data is 2.5. The maximum individual change occurred was 53 bpm, and the lowest was – 11 bpm (decreased by 11 bpm from lying to standing). The number of patients consisting of these values had a low statistical power and the number of patient data at each time interval ranged from 11 to 6. Thus, examining the lying to standing changes may be difficult to make a definitive conclusion as the data is insufficient.
Blood pressure
Challenges exist in the measurement of blood pressure, as the values can range depending on the practitioner technique (i.e. manual vs automatic) and the device itself (Mansoor et al. 2016). When examining for a trend in blood pressure changes, there did not appear to be a large difference. However, the averages oversimplify the differences for each patient. The individual changes ranged from an increase of 37 mmHg to a decrease of 39 mmHg when measuring blood pressure from a lying to standing position; Table 4 displays the variance for individual patients.
Discussion
The study took data from sites that were following guidelines implemented prior to August 2016 and examined available patient charts for monitoring data. Temperature, respiration rate, and pulse have a distinct peak centred around 4 hours with a range of 3-6 hours. The temperature did not rise above 38˚C in any of the patients (n = 43) between pre-dose and six hours post-dose (for days 1 and 2). Heart rate and the temperature had a greater increase on the second day. It is important to monitor temperature as fever is a risk factor for agranulocytosis and Neuroleptic Malignant Syndrome (NMS) (Novartis Pharmaceuticals, 2010; Young et al., 1998). Temperature elevation (>38˚C) is associated to have a peak incidence within the first three weeks of treatment. However, it is usually benign and self-limiting (Novartis Pharmaceuticals, 2010; Young et al., 1998). Therefore, elevation of temperature is not a good reason to stop therapy when examining just that parameter (O’Brien, 2004).
The effect of clozapine on heart rate elevation is that it may be a risk factor for myocarditis and cardiomyopathy (Safferman et al., 1991). However, the reports of myocarditis are rare and tachycardia (bpm > 100) occurs in 25% of all patient (Novartis Pharmaceuticals, 2010). Most commonly, monitoring heart rate is used to assess compliance and mild elevations of pulse is not significant enough to discontinue therapy (O’Brien, 2004). However, when the heart rate increases 30 beats per minute from baseline it may be a significant health concern.
When examining the average respiratory rate, the rate did not increase by more than one breath/minute in day 1 or day 2. When monitoring breathing rate, the primary concern is oversedation and respiratory collapse (O’Brien, 2004). However, it is rare to occur but, should be monitored closely when benzodiazepines are involved or other central nervous system depressants (Novartis Pharmaceuticals, 2010).
Blood pressure monitoring is the most significant vital to measure during clozapine initiation. Orthostatic hypotension has a 9% incidence and is dose-related (Marinkovic et al 1994; Safferman et al., 1991). Also, orthostatic hypotension is most likely to occur during the initial dose titration (California Correctional Health Care Services, 2017). Approximately 1 in 3000 patients experience collapse and respiratory or cardiac arrest when on clozapine (Young et al., 1998). The most significant blood pressure change occurred on the second day (dose dependent). For example, the blood pressure changes ranged from an increase of 39mmHg to a decrease of 39 mmHg. Thus, blood pressure varied quite considerably and averaged data can oversimplify these changes. It may be a health concern when the blood pressure deviates greater than 20 mmHg from baseline. Due to the high incidence of orthostatic hypertension and considerable blood pressure fluctuations, it is of particular importance to measure blood pressure over any other parameter during the first two days of a clozapine start.
Heart rate also had the most change on the second day (dose-dependent) and occurred between 3.5-5.5 hours. The pulse was noted to increase steadily after administering clozapine. However, there was not enough data to show a statistical difference.
There are several limitations to this study that should be considered. Due to an under recording of vital sign data and lack of complete health practitioner charts, there is a shortfall of information. It may be of interest to do a prospective study to gain further insight prior to implementing any recommendation. Only ~1/4 of charts contained some form of vitals monitoring data. The charts used had incomplete vital recordings and patient background, and thus, there is likely to be bias in the data (only certain nurses recorded and properly filed the vital sign data). Furthermore, there was weak statistical power in the study (n = 43).
References
- Ackenheil, M. (1989). Clozapine—pharmacokinetic investigations and biochemical effects in man. Psychopharmacology, 99(1), S32-S37.
- California Correctional Health Care Services. (2016). CCHCS/DHCS Care Guide: Retrieved from: http://www.cphcs.ca.gov/docs/careguides/Clozapine%20Care%20Guide.pdf
- Canadian Agency for Drugs and Technologies in Health. (2010). Clozapine Treatment of Hospitalized Patients: A Review of Clinical Practice Guidelines and Safety. Retrieved from https://www.cadth.ca/media/pdf/htis/dec_2010/L0234_Clozapine_Treatment_Hospitalized_Patients.
- Fraser Health Authority. (2015). Psychosis Treatment Optimization Program. Retrieved from: https://www.fraserhealth.ca/Service-Directory/Services/mental-health-and-substance-use/mentalhealth—community-services/psychosis-treatment-optimization-program#.W-Hzia2ZNn4
- Grohmann, R., Rüther, E., Sassim, N., & Schmidt, L. G. (1989). Adverse effects of clozapine. Psychopharmacology, 99 (Suppl), 101-104.
- Mansoor, K., Shahnawaz, S., Rasool, M., Chaudhry, H., Ahuja, G., & Shahnawaz, S. (2016). Automated versus manual blood pressure measurement: a randomized crossover trial in the emergency department of a tertiary care hospital in Karachi, Pakistan: are third world countries ready for the change?. Open access Macedonian journal of medical sciences, 4(3), 404.
- Marinkovic, D., Timotijevic, I., Babinski, T., Totic, S., & Paunovic, V. R. (1994). The sideeffects of clozapine: a four year follow-up study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 18(3), 537-544.
- McEvoy, J. P., Lieberman, J. A., Stroup, T. S., Davis, S. M., Meltzer, H. Y., Rosenheck, R. A.,… & Severe, J. (2006). Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry, 163(4), 600-610.
- Merrill, D. B., Ahmari, S. E., Bradford, J. M. E., & Lieberman, J. A. (2006). Myocarditis during clozapine treatment. American Journal of Psychiatry, 163(2), 204-208.
- Miller, D. D. (2000). Review and management of clozapine side effects. The Journal of clinical psychiatry.
- Novartis Pharmaceuticals. (2003). Out-patient initiation guidelines for Clozaril CD. East Hanover (NJ). Novartis Pharmaceuticals. (2010). East Hanover, New Jersey: Retrieved from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019758s062lbl.pdf
- O’Brien, A. (2004). Starting Clozapine in the Community. CNS drugs, 18(13), 845-852.
- Safferman, A., Lieberman, J. A., Kane, J. M., Szymanski, S., & Kinon, B. (1991). Update on the clinical efficacy and side effects of clozapine. Schizophrenia bulletin, 17(2), 247-261.
- Taylor, D., Paton, C., & Kapur, S. (2015). The Maudsley prescribing guidelines in psychiatry. John Wiley & Sons.
- Teva Pharmaceuticals. (2015). Example Titration Schedule. Retrieved from: http://tevaclozapine.com/documents/TitrationSalesAid_10601_Final.pdf
- Young, C. R., Bowers Jr, M. B., & Mazure, C. M. (1998). Management of the adverse effects of clozapine. Schizophrenia Bulletin, 24(3), 381.