Abstract
Wildlife cameras allow conservation scientists to monitor wildlife. However, there are performance limitations associated with wildlife cameras that must be understood prior to their use. This study compared two wildlife camera models, the Spypoint Solar Trail and the Reconyx Hyperfire 2, on behalf of Calgary Captured, a collaborative project between the Miistakis Institute and the City of Calgary to determine wildlife occupancy in Calgary’s Natural Area Parks. The camera models were set up in pairs at 10 sites. There was no significant difference in detections of white-tailed deer (Odocoileus virginianus) or coyotes (Canis latrans) by either model, but the Reconyx cameras successfully captured two species that the Spypoint model failed to detect: bobcat (Lynx rufus) and deer mouse (Peromyscus maniculatus). The Reconyx cameras had fewer trap days because the Nickel Metal Hydride (NiMH) batteries supplied by Calgary Captured consistently failed in cold weather, whereas the Spypoint cameras’ solar panels continued to function through the duration of the study. Despite having had fewer trap days, the Reconyx cameras captured more species than did the Spypoint cameras. There was no significant difference in the number of false positives and false negatives produced by the two models, but only the Spypoint cameras produced malfunctioned images. For projects like Calgary Captured, the Reconyx Hyperfire 2 is a more effective camera model than the Spypoint Solar Trail because it captures more species and is less prone to malfunction. The results of this study also highlight the importance of choosing appropriate batteries and settings for the camera model in question to ensure successful use.
Introduction
Determining the distribution of wildlife through wildlife surveys is essential to conservation (Ahumada, Hurtado, & Lizcano, 2013; O’Brien, Baillie, Krueger, & Cuke, 2010). Traditional wildlife survey methods such as scat and track transects can be expensive and time-consuming, and often result in misidentification or failure to detect species, hampering conservation efforts (Mackenzie, 2005). It is therefore important to select appropriate survey methods that avoid these biases (Miller, Nichols, McClintock, Campbell Grant, Bailey, & Weir, 2011). Wildlife cameras overcome some of these shortcomings by collecting data continuously over a long period of time and by easing photographic identification of wildlife, thereby increasing the survey period and reducing the probability of detection error (Vine, Crowther, Lapige, Dickman, Mooney, Piggot, & English, 2009). Vine et al. (2009) compared camera traps, hair traps, scat from bait stations, and spotlighting to determine the best survey method for a low-density red fox (Vulpes vulpes) population and found that camera traps provided the most consistent and univocal results, minimizing bias.
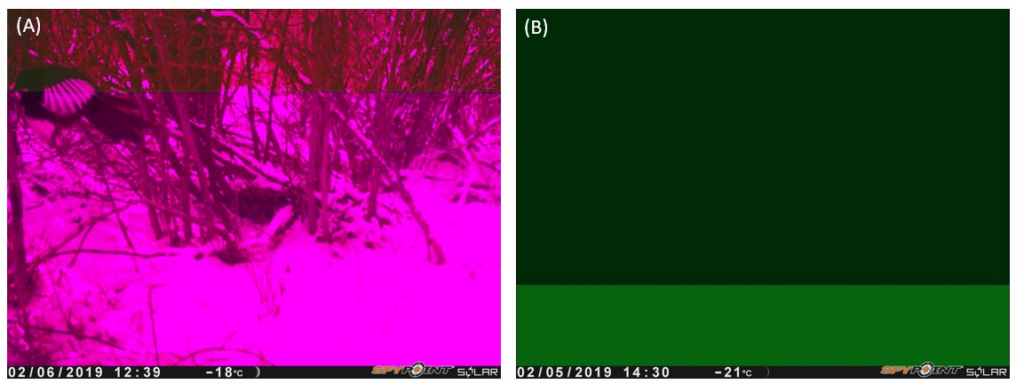
Despite these benefits, wildlife cameras have performance limitations and not all wildlife cameras perform equally (Meek, Ballard, & Fleming, 2015). Broadly speaking, there are performance disparities between “professional” and “recreational” camera models. Professional models are designed for research and incorporate higher-quality components (e.g.: sensors, camera lens, etc.), have more customization options, and are thus more expensive, whereas recreational models are intended for use by the general public and have limited features, resulting in a more affordable and user-friendly product (Newey, Davidson, Nazir, Fairhurst, Verdicchio, Irvine, & van der Wal, 2015). One study completed by Newey et al. (2015) found that recreational camera models are less reliable than professional models and produced a greater number of false positive (blank) images and false negative (missed detection) data. A false positive occurs when a camera takes a photo in the absence of wildlife. False positives can present issues because they consume memory, batteries, and time processing images (Heiniger & Gillespie, 2018). Newey et al. (2015) also note that recreational cameras are prone to malfunction, which also take up memory and drain batteries, possibly leading to data loss. For the purposes of this study, a malfunction is defined as an image that is colour-stained (Figure 1). Sometimes, wildlife can be identified from malfunctioned images, but in more serious malfunctions, the colour stain completely blocks the image, making identification impossible, as seen in Figure 1B.
Researchers also need to consider the specifications of wildlife cameras as differing specifications can result in highly variable data (Meek et al., 2015, p. 3). While countless studies have used wildlife cameras, few have considered the differences between models (Rovero, Zimmermann, Berzi, & Meek, 2013; Trolliet et al., 2013; Meek et al., 2015). A study will typically use only one model, so it is imperative that the selected camera has the minimum specifications required to fulfil the study’s needs. There can also be substantial variation between different units of the same model (Hughson, Darby, & Dungan, 2010), as camera set-up, settings, and the quantity of cameras used can alter the likelihood of detecting certain species (Rovero et al., 2013; Wellington, Bottom, Merrill, & Litvaitis, 2014; Meek, Ballard, & Falzon, 2016). Therefore, it is crucial that researchers consider not only which model, but also which settings to use (Driessen, Jarman, Troy, & Callander, 2017).
The purpose of this study is to examine differences between two wildlife camera models, the Spypoint Solar Trail and Reconyx Hyperfire 2, on behalf of Calgary Captured. Calgary Captured is a collaborative project between the City of Calgary and the Miistakis Institute to determine wildlife occupancy in Calgary’s Natural Area Parks using wildlife cameras (Miistakis Institute, 2017). Initially, Calgary Captured used Spypoint Solar Trail cameras, a recreational model as stated by its manual. However, after a period of two years, Calgary Captured began using the Reconyx Hyperfire 2, a professional model, following concerns from Calgary Captured over potential false negatives produced by the Spypoint models (Nicole Kahal, pers. comm.). It is important to understand the variation in performance between these different camera models to avoid making inaccurate conclusions about their results (Heiniger & Gillespie, 2018). Therefore, this study quantifies the difference in detection efficacy between the two models so that the Calgary Captured project can compare the data collected by the Spypoint cameras with those collected by the Reconyx cameras.
Methods
Study area
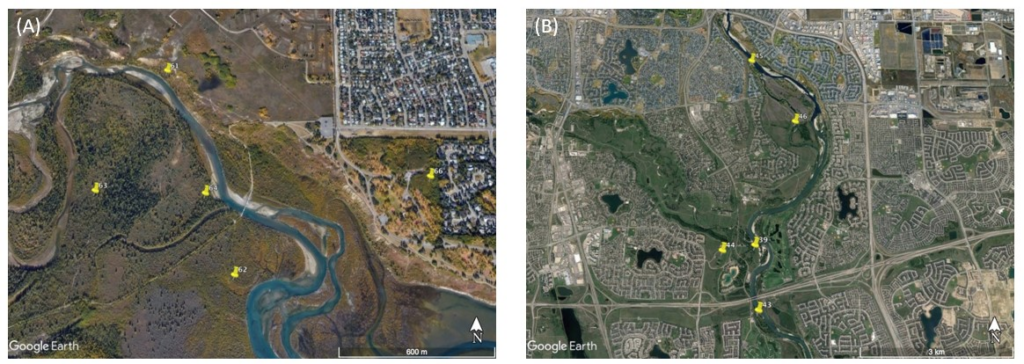
10 Spypoint and 10 Reconyx wildlife cameras were placed in pairs at 10 sites: four sites in Weaselhead Flats (Figure 2A), one site in North Glenmore Park (Figure 2A), and five sites in Fish Creek Provincial Park (Figure 2B). Weaselhead Flats and North Glenmore Park are adjacent to each other, and Fish Creek Provincial Park is located approximately 15 km southeast of Weaselhead Flats. All the parks used in this study have similar habitats consisting of riparian zones and aspen poplar, balsam poplar, and white spruce forest. The sites used for this experiment are 10 of the existing 69 Calgary Captured camera sites, which are systematically located near game trails and away from human trails in a 1km by 1km grid, except in Weaselhead Flats, where additional cameras were placed by Calgary Captured, resulting in a higher camera density (N. Kahal, pers. comm.).
Camera set-up
One Spypoint Solar Trail camera and one Reconyx Hyperfire 2 camera were placed together at each site. An ideal setup would place both cameras within 1 m directly next to each other (Hughson et al., 2010; Meek et al., 2015). However, there was rarely a pair of neighbouring trees close enough to allow for this setup. As a consequence, both cameras were placed on the same tree at nine sites, with the Spypoint camera placed above the Reconyx camera so that the Spypoint’s solar panel would not be obstructed by the Reconyx camera (Figure 3A). Site 62 was the exception and the cameras there were placed on neighbouring trees (Figure 3B). Camera height was measured by distance from the centre of the Fresnel lens to the ground (Table 1). Information on the specifications and settings of the cameras used in this study can be found in Table 2.

Data collection and processing
Memory card collection took place once a week, alternating between the Fish Creek Provincial Park sites and the Weaselhead Flats/North Glenmore Park sites. As a control measure, and to quantify false negatives, “walk-bys” were performed each time a camera was checked, during which the principal researcher walked past each camera three times, each time at three different speeds: slow (0.5m/s-0.6m/s), medium (0.9m/s-1.3m/s), and fast (1.5m/s-3.1m/s). This procedure was not performed at sites 43, 44, and 61 due to safety concerns.
The images captured by the cameras were processed using Microsoft Access and sorted into trap events, which consist of all the photos taken of an animal during its time in front of the cameras (Meek, Ballard, Claridge, Kays, Moseby, O’Brien, O’Connell, …, & Townsend, 2014). The Reconyx cameras captured three pictures for every detection while the Spypoint cameras took one picture for every detection, so the two models’ performances were compared using the number of events instead of the number of detections. Animals were identified by species for each event. Image processing was conducted on a secure computer at the Miistakis Institute office in compliance with the Alberta Freedom of Information and Protection of Privacy (FOIP) Act (Province of Alberta, 2019).
The cameras in Weaselhead Flats/North Glenmore Park wereactive from 15 January 2019 to 3 March 2019, and those in Fish Creek Provincial Park were active from 27 January 2019 to 8 March 2019. Due to the cold weather, the NiMH batteries in some Reconyx units repeatedly lost power, reducing the number of trap nights for those cameras (Table 1). Initially, one Spypoint camera was not working and had to be replaced with a different unit (site 64), reducing its number of trap nights (Table 1). To control for these differences in trap nights, the data were standardized to the number of trap events per 100 trap nights (Kelly & Holub, 2008):
\textit{RAI}=\frac{\textit{no. of events}\times100}{\textit{no. of nights}}This is the same standard used by the Miistakis Institute and is referred to in their documents as the relative abundance index, or RAI (Miistakis Institute, 2017). Throughout this paper, the number of trap events per 100 trap nights will be abbreviated to RAI.
Microsoft Excel was used to calculate RAI values for each species. To increase sample size, all bird species were placed into one category (“bird”). Because the data were not normally distributed, the non-parametric Wilcoxon signed-rank test was used for all comparisons. JMP (version 14.1.0) was used for statistical analyses and graphing.
Results
Throughout the study, nighttime temperatures often fell below the -18° operating limit specified for the NiMH batteries used in the Reconyx models. Consequently, only four of the ten Reconyx cameras functioned for the entire duration of the study. Furthermore, the camera locks were often frozen, which made changing the Reconyx cameras’ batteries impossible, exacerbating the problem. As a result, a substantial amount of Reconyx data were lost, which may have impacted the study.
Species detected
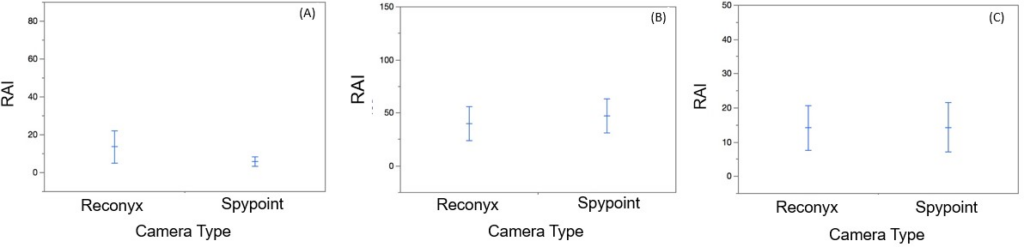
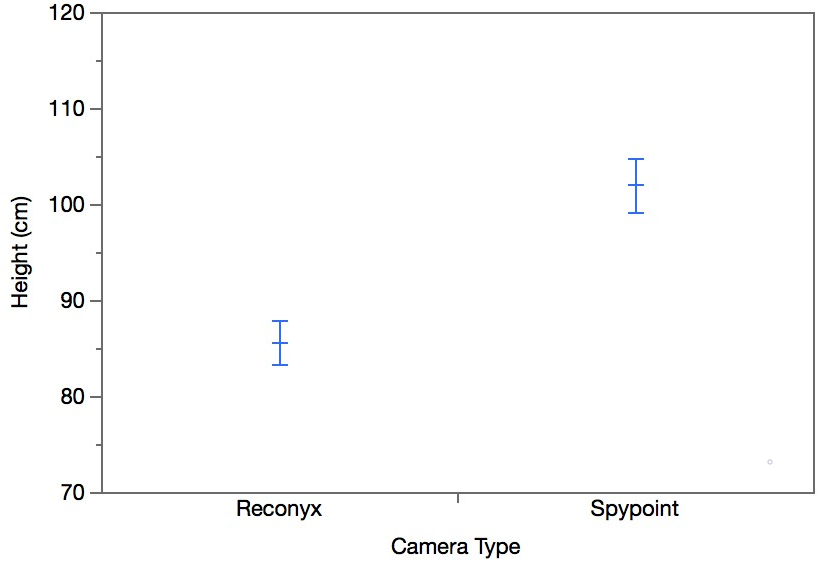
Nine species were detected during the study (Table 3). White-tailed deer was the most commonly detected species. Bobcat and deer mouse were detected only by the Reconyx cameras. There was no significant difference between the Reconyx and Spypoint cameras with regards to number of coyote detections (W=-7.00, p=0.31; Figure 4A) or deer detections (W=12.50, p=0.44; Figure 4B). Detection of other species did not take place at enough sites to allow for any statistical analysis (Table 3). Taking all species detections into consideration, there was no significant difference between Reconyx and Spypoint models (W=-0.13, p= 0.90; Figure 4C). It should be noted that at every site the camera height of the Spypoint cameras was significantly higher than that of the Reconyx cameras (W=3.44, p=0.0006; Figure 5).
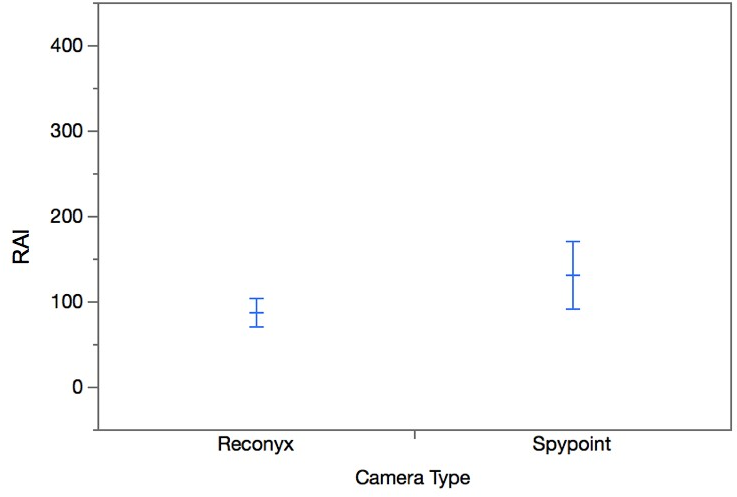
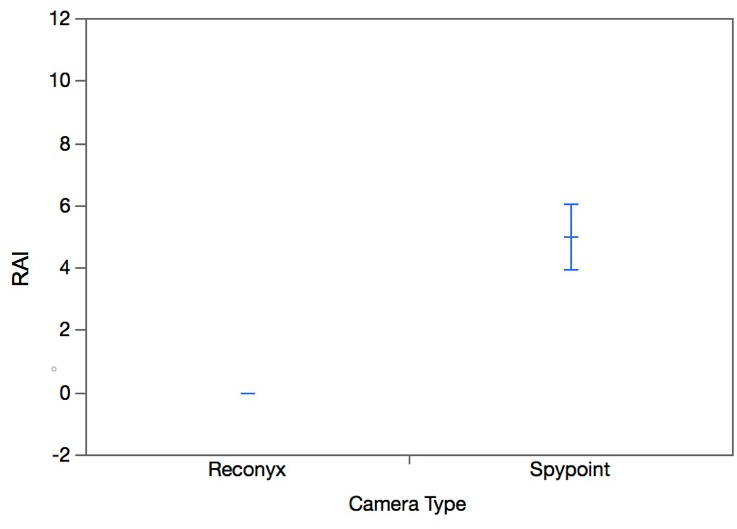
False positives and malfunctions
All of the cameras in this study collected false positives. There was no significant difference between the number of false positives collected by the Reconyx and Spypoint cameras (W=-15.50, p=0.13; Figure 6). There was less variation in the number of false positives collected by each Reconyx camera compared to the Spypoint cameras (Figure 6). Only the Spypoint cameras produced malfunctions. There was a significant difference between the number of malfunctions in the Spypoint cameras and Reconyx cameras (W=27.00, p=0.0039; Figure 7).
False negatives
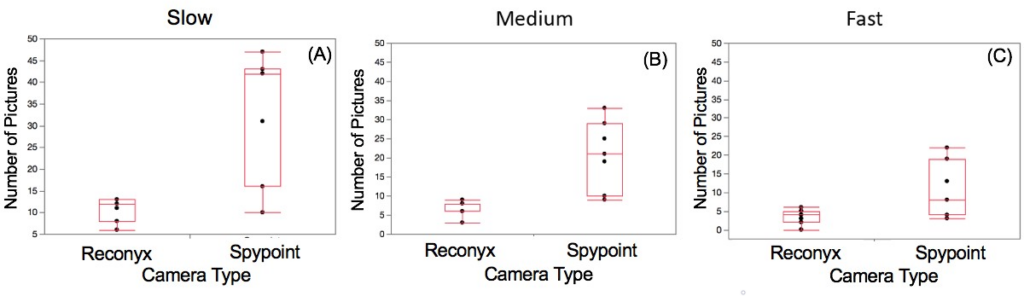
No significant difference was found between the number of pictures taken by the Reconyx and Spypoint cameras during the walk-by tests at slow (W=3.00, p=0.66), medium (W = 0.00, p = 1.00), or fast speeds (W=-1.00, p=0.89; Figure 8). One Reconyx camera failed to take any pictures during one of the fast walk-by tests. There was less variation in the number of pictures taken at each of the three walk-by speeds by the Reconyx cameras than by the Spypoint cameras (Figure 8).
Discussion
Over the course of the study, the Reconyx cameras had fewer trap nights than did the Spypoint cameras because their batteries failed in the cold weather. Despite this, the Reconyx cameras still detected more species than did the Spypoint cameras. While the RAI allows for comparison of data from cameras with differing numbers of trap nights, the Spypoint cameras should have a greater probability of detecting wildlife because they were functional for a greater period of time (Rovero et al., 2013). Thus, based on the number of trap nights alone, the Spypoint cameras should detect more species than the Reconyx cameras. The fact that the Reconyx cameras detected more species in fewer trap nights suggests that the model has greater detection ability.
The two species detected only by Reconyx cameras were bobcat (Lynx rufus) and deer mouse (Peromyscus maniculatus). Although Reconyx cameras have a reputation for being better able to detect smaller mammals (Kelly & Holub, 2008; Wellington et al., 2014) than the game species (e.g., deer) Spypoint cameras were designed to detect, there is little evidence in the literature to suggest that this is actually the case (Driessen et al., 2014). It was suggested that the reason the Spypoint cameras had lower than expected detection rates of bobcat was because bobcats move too quickly to be effectively detected (N. Kahal, pers. comm.). However, the walk-by tests did not find a significant difference in the number of pictures taken between the two camera models. In fact, the only camera that failed to take any photos during a fast walk-by test was a Reconyx camera.
Camera height has been found to account for variation in detection of species (Meek et al., 2016). To avoid blocking the Spypoint cameras’ solar panels, the Reconyx cameras were placed below the Spypoint cameras at nine sites. Meek et al. (2016) found that cameras placed 300 cm above the ground were less likely to detect species like red foxes and wild dogs (Canis familiaris) than those placed 210 cm lower at 90 cm. The greatest camera height in this study was 118 cm and the greatest difference in height between the two cameras at any site was only 31.25 cm. Moreover, there was no significant difference between the Reconyx and Spypoint cameras in the number of detections of coyote, a species comparable in size to wild dogs. The Spypoint cameras were also able to detect other small mammals such as Eastern grey squirrels and snowshoe hares, which are smaller than bobcats. Therefore, there was little indication that height difference was the reason the Reconyx cameras detected more species than did the Spypoint cameras. Camera placement is affected by many variables such as camera angle, distance from wildlife trails, and slope of the ground between the camera and wildlife trail (Meek et al., 2016). In this study, slope and camera angle may have affected species detections, although data for those variables were not collected so they could not be included in the analysis.
Camera settings such as trigger speed can affect the number of blank (false positive) images taken. If the trigger speed is too slow, it is possible for an animal to move through the field of view before a photo is taken (Wellington et al., 2014). In this study, the Reconyx cameras had a slower trigger speed than the Spypoint cameras, so the Reconyx cameras should have produced more false positives, which was not the case.
Only the Spypoint cameras produced malfunctions. This supports Newey et al.’s (2015) results showing that recreational quality cameras have performance limitations. Malfunctions negatively impact the quality of data because researchers often cannot identify the species that triggered the camera detection. Thus, susceptibility to malfunction should be considered when selecting a camera model for a study.
It was necessary to consider the variation in the number of trap nights in this study to be able to compare the efficacy of the two camera models. There are a variety of methods that have been used in the literature to standardize camera trap data (e.g. Carbone, Christie, Conforti, Franklin, Ginsberg, Griffiths, …, Sharuddin, 2001; O’Brien, Kinnaird, & Wibisono, 2003; O’Brien et al., 2010). This study used the method of Kelly & Holub (2008) to calculate RAIs for consistency with the methodology used by the Miistakis Institute (2017). Sollman, Mohamed, Samejima, & Wilting (2013) criticizes the use of RAIs because factors such as encounter rates, home ranges, trap setup, and repeated surveys all affect data quality, so using RAIs to determine wildlife abundance without considering these factors can return biased results. In this study, the RAI values were not used to extrapolate wildlife abundances, but rather were used to compare data collected by wildlife cameras that operated for different numbers of trap nights. Using the RAI values for this purpose precluded much of the biases described by Sollman et al. (2013).
Conclusions
Despite operating for fewer trap nights, the Reconyx cameras detected two species that were not detected by the Spypoint cameras. Because the goal of the Calgary Captured project is to document wildlife occupancy in the city, increased species detection is important. Moreover, the Reconyx cameras did not produce malfunctioned images, whereas the Spypoint cameras did. While the Reconyx cameras performed well, the NiMH batteries used did not. Lithium batteries with a specified operating limit of -40° have been used in other winter camera trap studies in Alberta without issue (L. Gould, pers. comm.; K. Anderson, pers. comm.) and may address this problem. Notwithstanding the battery issues in cold weather, Reconyx appears to be the better model in meeting the goals of Calgary Captured because it did not malfunction and was able to detect more species than did the Spypoint cameras despite operating for fewer trap nights
The advantages of using wildlife cameras over traditional survey methods include reduced identification errors and false negatives (Vine et al., 2009). Nonetheless, researchers need to be aware of wildlife cameras’ limitations and ensure the technology they select is suitable for the goals of their projects (Meek et al., 2015; Newey et al., 2015).
Acknowledgements
The author would like to thank Dr Dorothy Hill “for spending countless hours working on this project with me”, Dr Melanie Rathburn “for helping me run statistical tests”, Nicole Kahal “for helping with camera set-up and for being my contact at the Miistakis Institute”, Calgary Captured “for lending me their sites and equipment and for trusting me to conduct the study”, and Dr Paul Meek (Government of New South Wales), Tyne Baker, and Karen Anderson (Alberta Environment and Parks) “for sharing their knowledge of wildlife cameras and for their advice”.
References
- Ahumada, J. A., Hurtado, J., & Lizcano, D. (2013). Monitoring the trends and status of tropical forest terrestrial vertebrate communities from camera trap data: a tool for conservation. PLoS One, 8(9). https://doi.org/10.1371/journal. pone.0073707
- Carbone, C., Christie, S., Conforti, K., Coulson, T., Franklin, N., Ginsberg, J. R., Griffiths, M., Holden, J., Kawnanishi, K., Kinnaird, M., Laidlaw, R., Lynam, A., Macdonald, D. W., Martyr, D., McDougal, C., Nath, L., O’Brien, T., Seidensticker, J., Smith, D. J. L., Sunquist, M., Tilson, R., & Wan Shahruddin, W. N. (2001). The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation, 4, 75-79. https://doi. org/10.1017/S1367943001001081
- Driessen, M. M., Jarman, P. J., Troy, S., & Callander, S. (2017). Animal detection vary among commonly used camera trap models. Wildlife Research, 44(4), 291-297. https://doi.org/10.1071/WR16228
- Heiniger, J. & Gillespie, G. (2018). High variation in camera trap-model sensitivity for surveying mammal species in Northern Australia. Wildlife Research, 45(7), 578-585. https://doi.org/10.1071/WR18078
- Hughson, D. L., Darby, N. W., & Dungan, J. D. (2010). Comparison of motion-activated cameras for wildlife investigation. California fish and game, 92(2), 101-109.
- Kelly, M. J. & Holub, E. L. (2008). Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Gile County, Virginia. Northeastern naturalist, 15(2), 249-262. https://doi.org/10.1656/1092-6194(2008)15[249:CTOCTS]2.0. CO;2
- MacKenzie, D. I. (2005). What are the issues with presence-absence data for wildlife managers? The Journal of Wildlife Management, 69(3), 849-860. https://doi.org/10.2193/0022-541X(2005)069[0849:WATIWP]2.0.CO2
- Meek, P. D., Ballard, G., Claridge, A., Kays, R., Moseby, K., O’Brien, T., O’Connell, A., Sanderson, J., Swann, D. E., Tobler, M., & Townsend, S. (2014). Recommended principles for reporting on camera trapping research. Biodiversity and Conservation, 23(9), 2321-2343. https://doi.org/10.1007/s10531-014-0712-8
- Meek, P. D., Ballard, G., & Fleming, P. J. S. (2015). The pitfalls of wildlife camera trapping as a survey tool in Australia. Australian Mammalogy, 37(1), 13-22. https://doi.org/10.1071/AM14023
- Meek, P. D., Ballard, G., & Falzon, G. (2016). The higher you go the less you will know: placing camera traps high to avoid theft will affect detection. Remote Sensing in Ecology and Conservation, 2(4), 204-211. https://doi.org/10.1002/rse2.28
- Miistakis Institute. (2017). City of Calgary: wildlife camera monitoring: summary statistic May-August 2017. Calgary, Alberta: Kahal, N., Lee, T., & Rondeau, K.
- Miller, D. A., Nichols, J. D., McClintock, B. T., Campbell Grant, E. H., Bailey, L. L., & Weir, L. A. (2011). Improving occupancy estimation when two types of observational error occur: non-detection and species misidentification. Ecology, 92(7), 1422-1428. https://doi.org/10.1890/10-1396.1
- Newey, S., Davidson, P., Nazir, S., Fairhurst, G., Verdicchio, F., Irvine, R. J., & van der Wal, R. (2015). Limitations of recreational camera traps for wildlife management and conservation research: a practitioner’s perspective. Ambio, 44, 625-635. https://doi.org/10.1007/s13280-015-0713-1
- O’Brien, T. G., Kinnaird, M. F., Wibisono, H. T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation, 6, 131-139. https://doi.org/10.1017/S1367943003003172
- O’Brien, T. G., Baillie, J. E. M., Krueger, L., Cuke, M. (2010). The wildlife picture index: monitoring top trophic levels. Animal Conservation, 13, 335-343. https://doi.org/doi:10.1111/j.1469-1795.2010.00357.x
- Reconyx (2018). Hyperfire 2 Outdoor Series Camera: User Manual. Retrieved January 29, 2019 from http://images.reconyx.com/file/HyperFire2PROUserGuide2018_04_24_v3.pdf
- Rovero, F., Zimmerman, F., Berzi, D., & Meek, P. (2013). “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife applications. The Italian Journal of Mammalogy, 24(2), 148-156. https://doi.org/10.4404/hystrix-24.2-8789
- Sollman, R., Mohamed, A., Samejima, H., & Wilting, A. (2013). Risky business or simple solution – relative abundance indices from camera-trapping. Biological Conservation, 159, 405-412. https://doi.org/10.1016/j.biocon.2012.12.025
- Spypoint. (n.d.). User Manual: Solar Trail Camera. Retrieved January 29, 2019 from https://www.spypoint.com/manuals/Manual_SOLAR_EN.pdf
- Trolliet, F., Huynen, M., Vermeulen, C., & Hambuckers, A. (2014). Use of camera traps for wildlife studies: a review. BASE, 18(3), 446-454.
- Vine, S. J., Crowther, M., S., Lapidge, S. J., Dickman, C. R., Mooney, N., Piggott, M. P., & English, A. W. (2009). Comparison of methods to detect rare and cryptic species: a case study using the red fox (Vulpes vulpes). Wildlife Research, 36, 436-446. https://doi.org/10.1071/WR08069
- Wellington, K., Bottom, C., Merrill, C., & Litvaitis, J. A. (2014). Identifying performance differences among trail cameras used to monitor forest mammals. Wildlife Society Bulletin, 38(3), 634-638. https://doi.org/10.1002/wsb.425
